帕金森病(PD)是一种主要包括运动迟缓和静止性震颤等临床表现的神经退行性疾病,其发病率随年龄增长而升高[1-3]。PD的神经病理学标志是黑质(SN)致密部(SNc)表现出多巴胺能神经元丢失,以及路易小体形成[3]。慢性神经炎症是PD的主要特征[4]。PD病人SN区存在着小胶质细胞和星形胶质细胞激活,且存在着铁沉积[5-6]。除了SN,苍白球(GP)也是铁代谢的关键脑区[7]。PD病人GP铁含量显著高于健康对照[6, 8]。苍白球外侧部(GPe)是中央基底神经节核团。GPe神经元具有调节或维持正在进行的动作的能力。此外,GPe神经元参与了非运动功能,包括学习、睡眠以及对感觉和奖励线索的处理等[9-13]。GPe神经元种类复杂[14-15]。在1-甲基-4-苯基-1, 2, 3, 6-四氢吡啶(MPTP)制备的PD小鼠模型中,对SN胶质细胞的激活与铁沉积研究较多,但GPe是否存在胶质细胞的激活和铁沉积并没有报道。本研究采用慢性MPTP模型来模拟PD的发病进程,探究PD状态下GPe的炎症反应与铁沉积变化。
1 材料与方法 1.1 实验材料 1.1.1 动物来源及饲养SPF级8周龄C57BL/6小鼠,由江苏集萃药康公司提供。小鼠饲养在自由饮水取食、昼夜光照循环(12 h/12 h)、室温(20±2)℃、湿度(50±5)%的动物室,1周后开始实验。实验动物使用符合青岛大学医学部伦理委员会动物伦理学要求。
1.1.2 主要试剂及来源MPTP购自美国Sigma公司,神经胶质酸性蛋白(GFAP)抗体、离子钙结合衔接分子-1(IBA-1)抗体均购于美国Cell Signaling Technology公司,酪氨酸羟化酶(TH)抗体、山羊抗兔荧光二抗均购于美国Thermo Fisher Scientific公司。
1.2 实验方法 1.2.1 动物分组及处理将27只小鼠随机分为对照组(13只)和MPTP组(14只)。MPTP组小鼠腹腔注射质量浓度为1.8 g/L的MPTP(18 mg/kg体质量),对照组腹腔注射等体积生理盐水,每周2次,持续5周。
1.2.2 小鼠爬杆实验采用瑞沃德(RWD)公司提供的小鼠爬杆装置进行实验。爬杆为直径0.8 cm、长50.0 cm、顶端带有直径3.0 cm的圆球的木杆,表面以医用胶带缠绕防滑。整个装置置于塑料盒中,实验开始前用新垫料铺于底部,并没过底座。实验前1 d,对小鼠进行适应性训练,正式实验时将小鼠放置于杆顶端,头部朝上,记录小鼠掉头时间,取3次实验平均值进行统计分析。
1.2.3 脑组织免疫荧光染色取小鼠脑组织使用冷冻切片机(Leica,CM1950))进行切片。切片之前将机器预冷至-20 ℃,将脑组织用包埋剂OCT(SakuraFinetek)包埋并固定,切成厚度为20 μm的脑片。将脑片置于40 g/L甲醛溶液中固定10 min;PBS(0.01 mol/L)漂洗3次,每次10 min;用PBST稀释的体积分数0.05驴血清溶液封闭脑片1 h,随后将脑片置于使用封闭液配制的一抗(TH(1∶1 000),IBA-1(1∶200),GFAP(1∶300))中,并置于4 ℃摇床上孵育过夜;PBS(0.01 mol/L)漂洗3次,每次10 min;加入二抗室温避光摇床孵育2 h,随后在每孔中加入DAPI染色液50 μL继续孵育5 min;PBS(0.01 mol/L)漂洗3次,每次10 min。将脑片平铺至洁净的病理防脱载玻片上,适当干燥后用体积分数0.70甘油封片,用Olympus数字病理切片扫描系统观察并扫描。分别计数每个高倍视野(400倍)内SN区和GPe区TH、IBA-1和GFAP阳性细胞总数并取平均值。
1.2.4 普鲁士蓝铁染色(Perl’s-DAB)实验将小鼠脑片置于40 g/L甲醛溶液中固定5 min,去离子水漂洗30 s;置于以等体积混合的20 g/L亚铁氰化钾溶液与20 g/L的HCl溶液室温摇床孵育30 min;PBS(0.01 mol/L)漂洗2次,每次5 min;体积分数0.03的H2O2-甲醇溶液室温摇床孵育20 min,以封闭内源性过氧化氢酶;用PBS(0.01 mol/L)漂洗脑片2次,每次5 min;然后将小鼠脑片置于DAB显色液中避光显色10 min,用双蒸水终止显色;将脑片平铺至洁净的病理防脱载玻片上,完全干燥后用中性树胶封片。用Olympus数字病理切片扫描系统在明场显微镜下进行观察并扫描。分别计数每个高倍视野(400倍)内SN区和GPe区的铁染色阳性细胞数目并取平均值。
1.3 统计学处理采用Prism 9软件进行统计学分析。计量资料数据以x ± s表示,两组均数比较采用独立样本t检验。以P<0.05为差异有统计学意义。
2 结果 2.1 两组小鼠运动功能和SN区TH阳性神经元计数比较爬杆实验结果显示,与对照组小鼠的掉头时间(1.66±0.51)s比较,MPTP组的(2.12±0.48)s明显增加,差异有统计学意义(t=2.42,P<0.05)。运动行为学实验结果表明,MPTP组小鼠出现运动功能障碍。免疫荧光染色检测结果表明,与对照组小鼠SN区TH阳性神经元总数(8 383.00±865.16)相比,MPTP组的(6 443.00±470.71)显著减少,差异有统计学意义(t=4.83,P<0.05)。提示PD小鼠模型造模成功。
2.2 两组小鼠SN区星形胶质细胞、小胶质细胞和铁沉积比较免疫荧光染色结果表明,与对照组小鼠SN区GFAP(200.00±67.94)和IBA-1(243.10±16.32)阳性细胞总数相比,MPTP组小鼠的GFAP(329.00±47.30)和IBA-1(293.10±25.31)显著升高,差异有统计学意义(t=3.82、4.07,P<0.05)。提示MPTP诱导的PD小鼠模型SN区星形胶质细胞和小胶质细胞被激活。Perl’s-DAB染色表明,与对照组小鼠SN区铁阳性细胞数目(2 087.00±277.12)相比,MPTP组的(2 743.00±238.18)显著增多,差异有统计学意义(t=4.40,P<0.05)。提示MPTP诱导的PD模型小鼠SN区出现铁沉积。见图 1。
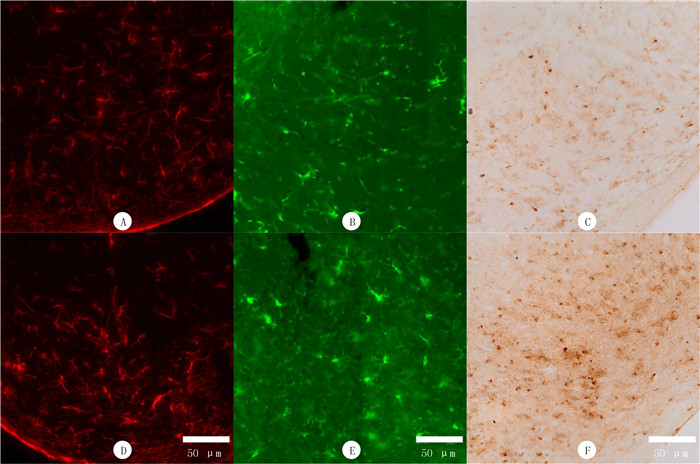 |
| A、B、D和E为免疫荧光染色;C和F为Perl’s-DAB染色。A、B和C为对照组,D、E和F为MPTP组。A和D为星形胶质细胞(GFAP)阳性染色,B和E为小胶质细胞(IBA-1)阳性染色,C和F为铁阳性细胞染色。 图 1 PD模型小鼠SN区免疫荧光染色和Perl’s-DAB染色观察 |
免疫荧光染色结果表明,与对照组小鼠GPe区IBA-1阳性细胞平均数(16.15±2.48)相比,MPTP组的(19.63±2.27)明显升高,差异有统计学意义(t=2.54,P<0.05)。提示GPe区小胶质细胞被激活。MPTP组小鼠GPe区GFAP阳性细胞数目与对照组比较,差异无显著性(t=1.35,P>0.05)。提示GPe区星形胶质细胞未被激活。Perl’s-DAB染色表明,MPTP组小鼠GPe区铁阳性细胞数目与对照组相比,差异无显著性(t=0.03,P>0.05)。提示MPTP诱导的PD模型小鼠GPe区并未出现铁沉积。见图 2。
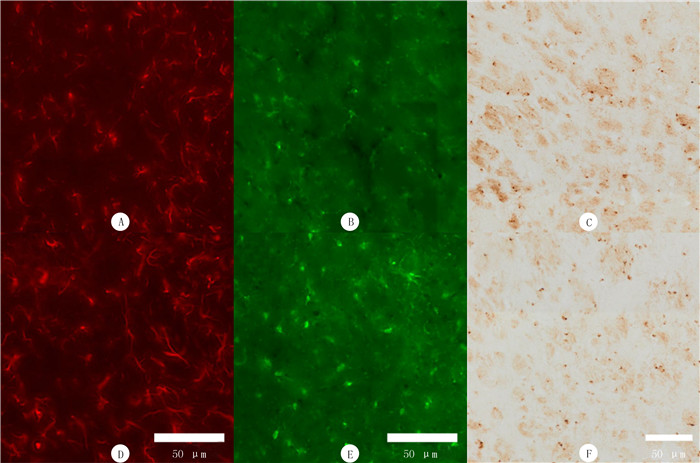 |
| A、B、D和E为免疫荧光染色;C和F为Perl’s-DAB染色。A、B和C为对照组,D、E和F为MPTP组。A和D为星形胶质细胞(GFAP)阳性染色,B和E为小胶质细胞(IBA-1)阳性染色,C和F为铁阳性细胞染色。 图 2 PD模型小鼠GPe区免疫荧光染色和Perl’s-DAB染色观察 |
在PD病人的SN中小胶质细胞激活先于神经元丢失,小胶质细胞激活可以加重神经炎症,进一步加剧神经元丢失,小胶质细胞激活可促进PD神经变性的进展[16-18]。有研究证明,GPe中的小清蛋白阳性神经元向黑质网状部具有直接投射,这种投射与小鼠的运动能力直接相关,并可以激活基底神经节中的核团[11]。同时,SN的多巴胺能神经元可以投射到GPe[19]。本文研究结果表明,SN多巴胺能神经元损伤时,该脑区的小胶质细胞和星形胶质细胞被激活,同时GPe小胶质细胞也被激活。已有文献报道,细胞内铁过载可加重内毒素或神经毒素对小胶质细胞的激活[20-21],且特别容易受到铁过载诱导的铁死亡的影响[22]。正常人脑组织中,GP铁含量较高[23-25]。我们推测,可能是由于GPe铁含量较高,所以当SN神经元损伤时,其投射至GPe的末梢受损,致小胶质细胞容易被激活。星形胶质细胞现在被认为是神经退行性疾病中的关键参与者[26-28]。相对于小胶质细胞,星形胶质细胞对铁的敏感性更低[22]。这可能是GPe未出现星形胶质细胞激活的原因之一。
磁共振成像技术显示,快速眼动睡眠行为障碍病人检测中仅SN发现铁含量升高,SN中铁沉积增加[6]。随着疾病发展,在其他大脑区域也出现铁沉积增加,包括壳核、GP等[8, 29]。最新研究表明,在多巴胺能神经元丧失之前,神经炎症首先发生在黑质-纹状体系统中,并同时出现铁沉积[20]。无论是在PD病人中还是在动物模型中,GPe铁沉积都要晚于SN铁沉积。本文仅在SN中观察到了铁沉积,而并未在GPe中观察到铁沉积。我们推测,本文PD模型小鼠GPe未出现铁沉积可能是由于造模时间较短,尚未引起GPe明显的铁沉积。小胶质细胞在SN中比其他脑区分布更丰富,与神经炎症密切相关,这导致神经元的区域特异性易感性[30-31]。这可能也是为什么SN更容易出现铁沉积,而GPe不容易出现铁沉积的原因之一。我们推测,延长造模时间可能会造成GPe铁沉积。
综上所述,经典慢性MPTP小鼠PD模型可以引起GPe中小胶质细胞的激活,不能引起星形胶质细胞的激活和铁沉积。后续会更换PD小鼠模型或延长造模时间,进行更深入的机制研究。本实验结果为研究模拟PD晚期时GPe胶质细胞激活和铁沉积提供了新的理论基础,为进一步探讨GPe脑区在PD发病机制中的作用提供了一定的实验依据。
| [1] |
BLOEM B R, OKUN M S, KLEIN C. Parkinson's disease[J]. Lancet, 2021, 397(10291): 2284-2303. DOI:10.1016/S0140-6736(21)00218-X |
| [2] |
DORSEY E R, CONSTANTINESCU R, THOMPSON J P, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030[J]. Neurology, 2007, 68(5): 384-386. DOI:10.1212/01.wnl.0000247740.47667.03 |
| [3] |
TOLOSA E, GARRIDO A, SCHOLZ S W, et al. Challenges in the diagnosis of Parkinson's disease[J]. The Lancet Neuro-logy, 2021, 20(5): 385-397. DOI:10.1016/S1474-4422(21)00030-2 |
| [4] |
KUSHWAHA R, SINHA A, MAKARAVA N, et al. Non-cell autonomous astrocyte-mediated neuronal toxicity in prion diseases[J]. Acta Neuropathologica Communications, 2021, 9(1): 22. DOI:10.1186/s40478-021-01123-8 |
| [5] |
MANDEL S, GRVNBLATT E, RIEDERER P, et al. Neuroprotective strategies in Parkinson's disease: an update on progress[J]. CNS Drugs, 2003, 17(10): 729-762. DOI:10.2165/00023210-200317100-00004 |
| [6] |
SUN J Y, LAI Z Y, MA J H, et al. Quantitative evaluation of iron content in idiopathic rapid eye movement sleep behavior disorder[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2020, 35(3): 478-485. DOI:10.1002/mds.27929 |
| [7] |
LI W, WU B, BATRACHENKO A, et al. Differential deve-lopmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan[J]. Human Brain Mapping, 2014, 35(6): 2698-2713. DOI:10.1002/hbm.22360 |
| [8] |
GUAN X J, XUAN M, GU Q Q, et al. Regionally progressive accumulation of iron in Parkinson's disease as measured by quantitative susceptibility mapping[J]. NMR in Biomedicine, 2017, 30(4). DOI:10.1002/nbm.3489 |
| [9] |
HEGEMAN D J, HONG E S, HERNÁNDEZ V M, et al. The external globus pallidus: progress and perspectives[J]. The European Journal of Neuroscience, 2016, 43(10): 1239-1265. DOI:10.1111/ejn.13196 |
| [10] |
COURTNEY C D, PAMUKCU A, CHAN C S. Cell and circuit complexity of the external globus pallidus[J]. Nature Neuroscience, 2023, 26(7): 1147-1159. DOI:10.1038/s41593-023-01368-7 |
| [11] |
LILASCHAROEN V, WANG E H, DO N, et al. Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits[J]. Nature Neuroscience, 2021, 24(4): 504-515. DOI:10.1038/s41593-021-00810-y |
| [12] |
ARISTIETA A, BARRESI M, AZIZPOUR LINDI S, et al. A disynaptic circuit in the globus pallidus controls locomotion inhibition[J]. Current Biology, 2021, 31(4): 707-721. e7. DOI:10.1016/j.cub.2020.11.019 |
| [13] |
THOMAS G E C, LEYLAND L A, SCHRAG A E, et al. Brain iron deposition is linked with cognitive severity in Parkinson's disease[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 2020, 91(4): 418-425. DOI:10.1136/jnnp-2019-322042 |
| [14] |
GITTIS A H, BERKE J D, BEVAN M D, et al. New roles for the external globus pallidus in basal Ganglia circuits and behavior[J]. The Journal of Neuroscience, 2014, 34(46): 15178-15183. DOI:10.1523/JNEUROSCI.3252-14.2014 |
| [15] |
HERNÁNDEZ V M, HEGEMAN D J, CUI Q L, et al. Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus[J]. The Journal of Neuroscience, 2015, 35(34): 11830-11847. DOI:10.1523/JNEUROSCI.4672-14.2015 |
| [16] |
SANCHEZ-GUAJARDO V, BARNUM C J, TANSEY M G, et al. Neuroimmunological processes in Parkinson's disease and their relation to α-synuclein: microglia as the referee between neuronal processes and peripheral immunity[J]. ASN Neuro, 2013, 5(2): 113-139. |
| [17] |
GELDERS G, BAEKELANDT V, VAN DER PERREN A. Linking neuroinflammation and neurodegeneration in Parkinson's disease[J]. Journal of Immunology Research, 2018, 2018: 4784268. |
| [18] |
JOHNSON M E, STECHER B, LABRIE V, et al. Triggers, facilitators, and aggravators: redefining Parkinson's disease pathogenesis[J]. Trends in Neurosciences, 2019, 42(1): 4-13. DOI:10.1016/j.tins.2018.09.007 |
| [19] |
BEUKEMA P, YEH F C, VERSTYNEN T. In vivo characterization of the connectivity and subcomponents of the human globus pallidus[J]. NeuroImage, 2015, 120: 382-393. DOI:10.1016/j.neuroimage.2015.07.031 |
| [20] |
MA X Z, CHEN L L, QU L, et al. Gut microbiota-induced CXCL1 elevation triggers early neuroinflammation in the substantia nigra of Parkinsonian mice[J]. Acta Pharmacologica Sinica, 2024, 45(1): 52-65. DOI:10.1038/s41401-023-01147-x |
| [21] |
WANG J, SONG N, JIANG H, et al. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons[J]. Biochimica et Biophysica Acta, 2013, 1832(5): 618-625. DOI:10.1016/j.bbadis.2013.01.021 |
| [22] |
KENKHUIS B, BUSH A I, AYTON S. How iron can drive neurodegeneration[J]. Trends in Neurosciences, 2023, 46(5): 333-335. DOI:10.1016/j.tins.2023.02.003 |
| [23] |
MOCHIZUKI H, CHOONG C J, BABA K. Parkinson's di-sease and iron[J]. Journal of Neural Transmission, 2020, 127(2): 181-187. DOI:10.1007/s00702-020-02149-3 |
| [24] |
AQUINO D, BIZZI A, GRISOLI M, et al. Age-related iron deposition in the basal Ganglia: quantitative analysis in healthy subjects[J]. Radiology, 2009, 252(1): 165-172. DOI:10.1148/radiol.2522081399 |
| [25] |
HALLGREN B, SOURANDER P. The effect of age on the non-haemin iron in the human brain[J]. Journal of Neurochemistry, 1958, 3(1): 41-51. DOI:10.1111/j.1471-4159.1958.tb12607.x |
| [26] |
BRANDEBURA A N, PAUMIER A, ONUR T S, et al. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders[J]. Nature Reviews Neuroscience, 2023, 24(1): 23-39. DOI:10.1038/s41583-022-00641-1 |
| [27] |
LEITNER D F, CONNOR J R. Functional roles of transferrin in the brain[J]. Biochimica et Biophysica Acta, 2012, 1820(3): 393-402. DOI:10.1016/j.bbagen.2011.10.016 |
| [28] |
SONG N, WANG J, JIANG H, et al. Astroglial and micro-glial contributions to iron metabolism disturbance in Parkinson's disease[J]. Biochimica et Biophysica Acta Molecular Basis of Disease, 2018, 1864(3): 967-973. DOI:10.1016/j.bbadis.2018.01.008 |
| [29] |
LI K R, AVECILLAS-CHASIN J, NGUYEN T D, et al. Quantitative evaluation of brain iron accumulation in different stages of Parkinson's disease[J]. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging, 2022, 32(2): 363-371. DOI:10.1111/jon.12957 |
| [30] |
QIAN Z M, KE Y. Brain iron transport[J]. Biological Reviews of the Cambridge Philosophical Society, 2019, 94(5): 1672-1684. DOI:10.1111/brv.12521 |
| [31] |
KIM W G, MOHNEY R P, WILSON B, et al. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia[J]. The Journal of Neuroscience, 2000, 20(16): 6309-6316. DOI:10.1523/JNEUROSCI.20-16-06309.2000 |
 2024, Vol. 60
2024, Vol. 60

