帕金森病(PD)是一种常见的神经退行性疾病,其患病率随着年龄的增长而上升[1]。PD的神经病理学标志是黑质(SN)致密部(SNc)出现多巴胺能神经元丢失,以及路易小体的形成。PD被认为是一种多系统疾病,其临床主要表现为运动迟缓、静息性震颤、僵直、姿势和步态改变等运动症状,以及嗅觉减退、便秘、体位性低血压、记忆丧失、抑郁、疼痛和睡眠障碍等非运动症状[1-2]。苍白球(GP),又称为旧纹状体,分为内侧苍白球(GPi)和外侧苍白球(GPe),位于基底神经节的中心位置。GPe内95%的神经元为γ-氨基丁酸抑制性神经元,是基底神经节的主要输出核团,在间接通路中连接纹状体与丘脑底核,在运动功能调节方面发挥重要作用[3]。在PD中GPe功能障碍几乎与所有的运动和非运动症状有关。铁死亡是铁依赖性的脂质过氧化驱动的一种独特的细胞死亡方式[4]。Erastin和RSL3是两种经典的铁死亡诱导剂。Erastin通过抑制Xc-系统,促进细胞对胱氨酸的摄取减少,从而降低细胞内还原型谷胱甘肽(GSH)的水平;可以改变线粒体外膜的通透性,其作用靶点是电压依赖性阴离子通道(VDACs);还可以通过调节ACSL4来刺激脂质过氧化[5-11]。RSL3是另一种铁死亡诱导剂[12],它不依赖于VDACs或Xc-系统,而是通过与GPX4的结合导致后者失去活性,因此一般认为GPX4参与RSL3诱导的铁死亡,导致脂质过氧化物的积累增加[13-17]。在PD的体外和体内模型中,铁死亡已被证明是一种普遍的细胞死亡方式[18-19]。PD病人虽然存在苍白球铁沉积,但尚无研究报道是否存在铁死亡,GPe铁死亡是否与运动行为相关更无从可知[20]。本研究通过GPe立体定位注射铁死亡诱导剂RSL3或Erastin探究其对小鼠运动行为影响。
1 材料和方法 1.1 实验材料 1.1.1 实验动物与饲养SPF级雄性C57BL/6小鼠,8周龄,体质量(21±2)g,购自江苏集萃药康生物科技股份有限公司。将小鼠饲养于可自由饮水取食、室温(20±2)℃、湿度(50±5)%、昼夜循环光照(12 h/12 h)的清洁环境中。动物实验符合青岛大学动物伦理学要求。
1.1.2 主要试剂及来源Erastin购自美国Sigma公司,RSL3购自美国Selleck公司,ACSL4、GPX4一抗均购自英国abcam公司,Rabbit-Anti-β-actin一抗购自中国博奥森公司,Goat-Anti-Rabbit IgG二抗购自中国爱必信公司。
1.2 实验方法 1.2.1 动物分组与处理将小鼠随机分为对照组(8只)和实验组(Erastin组12只、RSL3组12只)。用异氟烷对小鼠进行全身麻醉,然后将其固定在脑立体定位仪上。将小鼠颅脑背侧皮肤切开,以体积分数0.03的过氧化氢溶液从颅骨表面擦洗,直至颅缝和前后囟清晰可见。调整确定坐标(右侧GPe立体定位坐标为前囟后0.35 mm、右旁开2.05 mm、深度3.70 mm)后,采用微量注射泵在两侧GPe注射200 nL生理盐水或铁死亡诱导剂(RSL3或Erastin,浓度均为10 μmol/L),流量为0.5 nL/s,注射结束后留针10 min再缓慢退针。注射两周后对小鼠进行行为学检测。
1.2.2 步态分析实验通过Cat Walk(Noldus)自动化步态测试,对小鼠的运动量和运动协调性进行评价。采用爪印的长度或宽度、爪印与玻璃板的接触面积、接触压强和步长等静态参数,还有摆动速度、站立时间、站立指数、悬空时间和行走周期时间等动态参数,评估运动能力。以正常步态比例、双足侧向间距、支撑力等为指标,评价运动协调能力及姿态稳定性。在收集数据时,要保证每只小鼠收集到最少3个有效循环,系统会自动地对所有参数进行记录,并取其平均值进行统计分析。
1.2.3 免疫印迹法检测铁死亡相关蛋白ACSL4与GPX4的表达行为学检测结束后,处死小鼠,取新鲜的GPe并测定其质量。将RIPA蛋白裂解液加入组织样品中(每毫克25 μL),机械破碎后将其放置在冰面上30 min,使其完全裂解。然后,以4 ℃、12 000 r/min离心25 min,取出上清,用BCA蛋白定量试剂盒对其进行测定。将处理好的蛋白样本进行聚丙烯酰胺凝胶电泳后转PVDF膜(0.45 μm)。用50 g/L脱脂奶粉在室温下封闭2 h,然后分别加入一抗GPX4 (1∶5 000)、ACSL4 (1∶5 000)和β-actin(1∶10 000)在4 ℃下孵育过夜,再加入山羊抗兔二抗(1∶10 000)室温孵育1 h。采用ECL法进行显影。使用Image J软件进行条带灰度值计算,并以目标蛋白与内参照β-actin条带灰度值之比为指标计算GPX4和ACSL4的相对表达量。
1.3 统计学处理应用Prism 6软件进行统计学分析。计量资料数据以x ± s表示,多组均数比较采用单因素方差分析(One-Way ANOVA),组间两两比较使用Turkey法。以P<0.05表示差异有统计学意义。
2 结果 2.1 铁死亡诱导剂对小鼠运动行为影响与对照组相比,Erastin组小鼠的右前肢摆动速度升高(F=5.433, q=4.039, P<0.05),右前肢步幅长度增加(F=7.486, q=3.693, P<0.05)。与对照组相比,RSL3组小鼠的右前肢的摆动速度升高、步幅长度增加(F=5.433、7.486, q=4.286、5.446, P<0.05、0.01),左前肢的摆动速度、身体速度均升高(F=4.106、4.745, q=3.966、4.329, P<0.05),右前肢的最大接触面积和脚印长度均明显增加(F= 6.540、6.465, q=4.286、5.012, P<0.01),右前肢最小强度明显减小(F=7.281, q=5.397, P<0.01),右后肢的身体速度升高、步幅长度增加(F=3.467、4.111, q=3.715、3.728, P<0.05),左后肢的身体速度升高、步幅长度增加(F=6.242、9.873, q=4.838、6.093, P<0.01、0.001)。见表 1。
| 表 1 小鼠GPe立体定位注射Erastin或RSL3的步态分析(n=12, x ± s) |
|
|
与对照组相比,Erastin组小鼠GPe中GPX4表达下降(F=6.207, q=3.847, P<0.05),ACSL4表达水平不变(F=19.740, q=1.419, P>0.05)。与对照组相比较,RSL3组小鼠GPe中GPX4的表达水平明显下降(F=6.207, q=4.618, P<0.05),ACSL4的表达水平则明显升高(F=19.740, q=7.735, P<0.01)。见图 1。
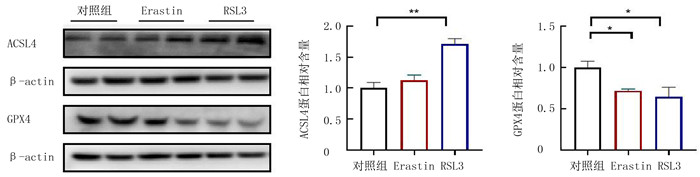 |
| 注:与对照组比较,*P<0.05,* *P<0.01。 图 1 GPe注射铁死亡诱导剂对ACSL4与GPX4蛋白表达的影响 |
正常生理状态下,基底神经节中铁含量丰富,以GP和SN铁含量最高,且随年龄增长而累积[21-24]。在PD疾病状态下,随着磁共振成像技术的进步,越来越多的证据表明SN中的铁含量高于正常[25]。有证据表明,PD病人SN中的铁含量与运动障碍相关[26]。有研究应用磁敏感定量成像(QSM)技术,分析早期PD组和晚期PD组病人的脑铁含量,发现PD不同阶段铁积累呈区域进行性模式,SNc的铁沉积只在疾病的早期阶段受到影响,而黑质网状部、红核和GP,特别是GPi部分,则在疾病的晚期阶段受到影响[27]。除了SN,在PD病人中GP铁含量也升高[28]。特发性快速眼动睡眠行为障碍(iRBD)是神经退行性疾病的早期征象。QSM评估iRBD病人SN中的铁含量增加,但是没有发现GP中的铁含量增加,这就说明PD病人GP铁沉积可能是相对较晚的疾病特征[29]。
GPe是基底神经节运动回路间接通路的关键组成部分,是连接纹状体到丘脑底核的关键[30-32]。最近的研究表明,GPe中的小清蛋白(PV)神经元直接投射到黑质网状部,与小鼠的运动能力直接相关,并可激活基底神经节中的多种核团,包括丘脑底核和GPi。运用化学遗传方法特异性灭活PV神经元时,神经元自发放电频率和小鼠运动功能明显降低,但是小鼠的逆转学习不受影响。在6-羟多巴胺诱导的PD模型小鼠中,利用光遗传技术特异性激活GPe的PV神经元可以显著提高小鼠在开阔场实验中的运动速度,提示其运动能力增强[33]。PV神经元是GPe占GABA神经元比例最高的神经元类型,快速高频放电的特性使其呈现高代谢需求,因此对氧化应激特别敏感[34]。有研究报道,在PD病人的尸检脑组织和6-羟多巴胺诱导的PD模型小鼠中,GPe中PV神经元的数量减少[35-36]。我们推测,PD时GPe铁沉积很可能通过诱导铁死亡造成PV神经元活动异常从而参与PD运动障碍。
本文实验结果表明,在GPe立体定位注射铁死亡诱导剂RSL3或Erastin两周后,GPX4蛋白表达水平明显下降,提示GPe中发生铁死亡,但行为学检测结果显示小鼠运动行为增强。注射RSL3两周后,ACSL4蛋白的表达水平明显升高,而Erastin组GPe中ACSL4蛋白没有变化。其原因可能是两种药物的作用靶点以及机制不同,Erastin主要是抑制Xc-系统,作用靶点在VDACs, 而RSL3主要是与GPX4结合。有研究表明,在神经退行性变初期神经元兴奋性增加[35-37]。我们推测,GPe注射铁死亡诱导剂后小鼠运动能力增强,可能与GPe神经元兴奋性增加有关,代表了GPe神经元损伤的初期。本实验中所使用的铁死亡诱导剂浓度较低、时间较短,虽然在小鼠的GPe中诱导了明显的铁死亡,但此时该脑区某些神经元兴奋性却是升高的而不是降低的,随着铁死亡诱导剂浓度增加或者时间的延长可能会损伤神经元而导致运动障碍。
综上所述,本研究进一步证实了GPe与小鼠运动行为相关,为临床上GPe神经元活动异常与PD运动障碍提供了新的实验证据。
| [1] |
TOLOSA E, GARRIDO A, SCHOLZ S W, et al. Challenges in the diagnosis of Parkinson's disease[J]. The Lancet Neuro-logy, 2021, 20(5): 385-397. DOI:10.1016/S1474-4422(21)00030-2 |
| [2] |
COSTA H N, ESTEVES A R, EMPADINHAS N, et al. Parkinson's disease: a multisystem disorder[J]. Neuroscience Bulletin, 2023, 39(1): 113-124. DOI:10.1007/s12264-022-00934-6 |
| [3] |
HEGEMAN D J, HONG E S, HERNÁNDEZ V M, et al. The external globus pallidus: progress and perspectives[J]. The European Journal of Neuroscience, 2016, 43(10): 1239-1265. DOI:10.1111/ejn.13196 |
| [4] |
STOCKWELL B R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications[J]. Cell, 2022, 185(14): 2401-2421. DOI:10.1016/j.cell.2022.06.003 |
| [5] |
DOLMA S, LESSNICK S L, HAHN W C, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells[J]. Cancer Cell, 2003, 3(3): 285-296. DOI:10.1016/S1535-6108(03)00050-3 |
| [6] |
YAN R, XIE E, LI Y, et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis[J]. Cell Research, 2022, 32(7): 687-690. DOI:10.1038/s41422-022-00642-w |
| [7] |
WANG L, LIU Y, DU T, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc[J]. Cell Death and Differentiation., 2020, 27(2): 662-675. DOI:10.1038/s41418-019-0380-z |
| [8] |
LI Y, ZENG X, LU D, YIN M, SHAN M, GAO Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis[J]. Human Reproduction, 2021, 36(4): 951-964. DOI:10.1093/humrep/deaa363 |
| [9] |
YAGODA N, VON RECHENBERG M, ZAGANJOR E, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels[J]. Nature, 2007, 447(7146): 864-868. |
| [10] |
ZHAO Y C, LI Y Q, ZHANG R F, et al. The role of erastin in ferroptosis and its prospects in cancer therapy[J]. OncoTargets and Therapy, 2020, 13: 5429-5441. DOI:10.2147/OTT.S254995 |
| [11] |
YUAN H, LI X M, ZHANG X Y, et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis[J]. Biochemical and Biophysical Research Communications, 2016, 478(3): 1338-1343. DOI:10.1016/j.bbrc.2016.08.124 |
| [12] |
YANG W S, STOCKWELL B R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells[J]. Che-mistry & Biology, 2008, 15(3): 234-245. |
| [13] |
SHIN D, KIM E H, LEE J, et al. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer[J]. Free Radical Biology and Medicine, 2018, 129: 454-462. DOI:10.1016/j.freeradbiomed.2018.10.426 |
| [14] |
YANG W S, KIM K J, GASCHLER M M, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(34): E4966-E4975. |
| [15] |
CHEFF D M, HUANG C, SCHOLZEN K C, et al. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1[J]. Redox Biology, 2023, 62: 102703. DOI:10.1016/j.redox.2023.102703 |
| [16] |
CUI Y, ZHANG Z, ZHOU X, et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression[J]. Journal of Neuroinflammation, 2021, 18(1): 249. DOI:10.1186/s12974-021-02231-x |
| [17] |
SUI X, ZHANG R, LIU S, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer[J]. Frontiers in Pharmacology, 2018, 9: 1371. DOI:10.3389/fphar.2018.01371 |
| [18] |
YAN H F, ZOU T, TUO Q Z, et al. Ferroptosis: mechanisms and links with diseases[J]. Signal Transduction and Targeted Therapy, 2021, 6(1): 49. DOI:10.1038/s41392-020-00428-9 |
| [19] |
VAN B D, GOUEL F, JONNEAUX A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC[J]. Neurobiology of Disease, 2016, 94: 169-178. DOI:10.1016/j.nbd.2016.05.011 |
| [20] |
ACOSTA-CABRONERO J, CARDENAS-BLANCO A, BETTS M J, et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson's disease[J]. Brain: a Journal of Neurology, 2017, 140(1): 118-131. DOI:10.1093/brain/aww278 |
| [21] |
THOMAS G E C, ZARKALI A, RYTEN M, et al. Regional brain iron and gene expression provide insights into neurodegeneration in Parkinson's disease[J]. Brain: A Journal of Neurology, 2021, 144(6): 1787-1798. DOI:10.1093/brain/awab084 |
| [22] |
WOLOZIN B, GOLTS N. Iron and Parkinson's disease[J]. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 2002, 8(1): 22-32. |
| [23] |
JIMÉNEZ-JIMÉNEZ F J, ALONSO-NAVARRO H, GARCÍA-MARTÍN E, et al. Biological fluid levels of iron and iron-rela-ted proteins in Parkinson's disease: review and meta-analysis[J]. European journal of neurology, 2021, 28(3): 1041-1055. DOI:10.1111/ene.14607 |
| [24] |
XU Y, HUANG X, GENG X, et al. Meta-analysis of iron metabolism markers levels of Parkinson's disease patients determined by fluid and MRI measurements[J]. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements, 2023, 78: 127190. DOI:10.1016/j.jtemb.2023.127190 |
| [25] |
LANGLEY J, HE N Y, HUDDLESTON D E, et al. Reproducible detection of nigral iron deposition in 2 Parkinson's di-sease cohorts[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2019, 34(3): 416-419. DOI:10.1002/mds.27608 |
| [26] |
MOCHIZUKI H, CHOONG C J, BABA K. Parkinson's di-sease and iron[J]. Journal of Neural Transmission, 2020, 127(2): 181-187. DOI:10.1007/s00702-020-02149-3 |
| [27] |
GUAN X J, XUAN M, GU Q Q, et al. Regionally progressive accumulation of iron in Parkinson's disease as measured by quantitative susceptibility mapping[J/OL]. NMR in Biomedicine, 2017, 30(4): 10.1002/nbm. 3489. doi: 10.1002/nbm.3489.
|
| [28] |
UCHIDA Y, KAN H, SAKURAI K, et al. Voxel-based quantitative susceptibility mapping in Parkinson's disease with mild cognitive impairment[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2019, 34(8): 1164-1173. DOI:10.1002/mds.27717 |
| [29] |
SUN J Y, LAI Z Y, MA J H, et al. Quantitative evaluation of iron content in idiopathic rapid eye movement sleep behavior disorder[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2020, 35(3): 478-485. DOI:10.1002/mds.27929 |
| [30] |
GITTIS A H, BERKE J D, BEVAN M D, et al. New roles for the external globus pallidus in basal ganglia circuits and behavior[J]. The Journal of neuroscience: the official journal of the Society for Neuroscience, 2014, 34(46): 15178-15183. DOI:10.1523/JNEUROSCI.3252-14.2014 |
| [31] |
DONG J, HAWES S, WU J, et al. Connectivity and functio-nality of the globus pallidus externa under normal conditions and Parkinson's disease[J]. Frontiers in neural circuits, 2021, 15: 645287. DOI:10.3389/fncir.2021.645287 |
| [32] |
HEGEMAN D J, HONG E S, HERNÁNDEZ V M, et al. The external globus pallidus: progress and perspectives[J]. The European JJournal of Neuroscience, 2016, 43(10): 1239-1265. DOI:10.1111/ejn.13196 |
| [33] |
LILASCHAROEN V, WANG E H, DO N, et al. Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits[J]. Nature Neuroscience, 2021, 24(4): 504-515. DOI:10.1038/s41593-021-00810-y |
| [34] |
FERNÁNDEZ-SUÁREZ D, CELORRIO M, LANCIEGO J L, et al. Loss of parvalbumin-positive neurons from the globus pallidus in animal models of Parkinson disease[J]. Journal of Neuropathology and Experimental Neurology, 2012, 71(11): 973-982. DOI:10.1097/NEN.0b013e3182717cba |
| [35] |
HARDMAN C D, HALLIDAY G M. The external globus pallidus in patients with Parkinson's disease and progressive supranuclear palsy[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 1999, 14(4): 626-633. DOI:10.1002/1531-8257(199907)14:4<626::AID-MDS1012>3.0.CO;2-U |
| [36] |
TOZZI A, SCIACCALUGA M, LOFFREDO V, et al. Dopamine-dependent early synaptic and motor dysfunctions induced by α-synuclein in the nigrostriatal circuit[J]. Brain: a Journal of Neurology, 2021, 144(11): 3477-3491. DOI:10.1093/brain/awab242 |
| [37] |
LASSER-KATZ E, SIMCHOVITZ A, CHIU W H, et al. Mutant α-synuclein overexpression induces stressless pacema-king in vagal motoneurons at risk in Parkinson's disease[J]. The Journal of Neuroscience, 2017, 37(1): 47-57. DOI:10.1523/JNEUROSCI.1079-16.2016 |
 2024, Vol. 60
2024, Vol. 60

