2. 山东第一医科大学附属成武医院内分泌科;
3. 山东第一医科大学附属成武医院心血管内科;
4. 青岛大学附属医院医学研究中心
随着生活水平的提高,2型糖尿病(T2DM)的患病率逐年增加。流行病学调查显示,T2DM病人非乙醇性脂肪肝(NAFL)的发生率高达49%~62%[1]。与单纯T2DM病人比较,合并NAFL的T2DM病人的血糖水平更高、波动更大、更难控制,心血管疾病的发病风险也增加。NAFL可能进一步进展为肝炎、肝硬化、肝衰竭和肝癌,严重威胁人类健康[2-3]。异荭草素(ISO)是一种木犀草素糖苷类黄酮化合物,其分子式为C21H20O11,分子量为448.38,微溶于甲醇溶剂。ISO广泛存在于竹茹、竹叶、西番莲、集花龙胆、葫芦果、满天星、决明子、黄荆、荞麦芽等多种植物的食用或药用部位[4-11]。研究表明,ISO具有抗炎、抗氧化、抗病毒等作用[12-14]。此外,ISO还具有保肝、降糖及调脂作用[6, 15-16]。但ISO的具体作用机制目前尚未有深入研究。本实验采用高脂高糖饲养及腹腔注射链脲佐菌素(STZ)方法构建T2DM合并NAFL小鼠模型,研究ISO的保肝作用并探讨其作用机制,以期为T2DM合并NAFL的治疗提供新思路。
1 材料与方法 1.1 主要材料SPF级4~5周龄雄性C57BL/6J小鼠,体质量(25±3)g,购自维通利华实验动物技术有限公司(许可证号SCXK(京)2016-0006)。ISO(纯度>98%),购自北京普天同创生物科技有限公司;STZ购自美国MedChemExpress公司;总胆固醇(TC)、三酰甘油(TG)、游离脂肪酸(FFA)、总抗氧化能力(T-AOC)、总超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、还原型谷胱甘肽(GSH)、丙二醛(MDA)比色法试剂盒购自武汉伊莱瑞特生物科技股份有限公司;转录因子NF-E2相关因子(Nrf-2)、血红素加氧酶-1(HO-1)、葡萄糖转运载体2(GLUT2)和β-actin抗体及二抗购自美国Cell Signaling Technology公司;显影液购自美国MILLIPORE公司。
1.2 实验方法 1.2.1 动物模型建立与药物干预SPF级雄性C57BL/6J小鼠60只适应性饲养1周,随机选取12只作为正常对照组,给予普通饲料喂养;其余48只小鼠使用60%的高脂饲料喂养4周,然后连续5次腹腔注射小剂量(30 mg/kg)STZ,1周后检测随机血糖连续2次大于16.7 mmol/L诊断为T2DM小鼠。再继续用60%的高脂饲料喂养4周,构建T2DM合并NAFL模型。将造模成功的T2DM合并NAFL小鼠随机分为模型对照组和ISO低、中、高剂量组,每组12只。ISO低、中、高剂量组分别按10、20、40 mg/kg剂量腹腔注射给药;正常对照组和模型对照组给予等量的5 g/L羧甲基纤维素钠腹腔注射。建模成功当天记作第0天,每周检测空腹血糖(FBG)水平,给药8周后处死小鼠,留取血及肝组织标本待检。
1.2.2 油红O染色观察肝组织脂肪样变性处死小鼠后立即取出部分肝脏组织,用OCT包埋剂包埋后冷冻切片,将冷冻切片复温干燥,置固定液中固定,自来水冲洗。入油红染液中浸染8~10 min,取出切片背景分化后浸入苏木精中复染3~5 min,纯水浸洗,分化液分化,蒸馏水冲洗,返蓝液返蓝,自来水浸洗,甘油明胶封片。光镜下采集图像并观察各组肝组织细胞脂滴沉积情况。
1.2.3 血清FBG检测末次干预后小鼠禁食12 h眼球采血收集血样,离心收集血清并进行分装,配制工作试剂,在全自动生化分析仪上设置相应参数,上样,全自动生化分析仪自动测定FBG值。
1.2.4 肝组织脂质和氧化应激指标检测新鲜肝脏组织用超声匀浆,取上清,采用BCA法检测蛋白浓度。按照比色试剂盒操作步骤检测TC、TG、FFA的含量以评估肝组织脂质水平,检测T-AOC、SOD、CAT、GSH、MDA水平以评估细胞内抗氧化状态。
1.2.5 Western blotting检测肝组织中Nrf-2、HO-1、GLUT2蛋白的表达用含蛋白酶抑制剂和磷酸酶抑制剂的裂解液裂解肝组织,提取总蛋白,采用BCA法测定总蛋白浓度。按每泳道50 μg蛋白量上样到SDS聚丙烯酰胺凝胶中,80 V恒压电泳10~15 min,120 V恒压电泳约1 h;将蛋白电转移至PVDF膜上,用50 g/L的牛奶封闭液封闭2.5 h;分别加入一抗(Nrf-2、HO-1、GLUT2的稀释比均为1∶1 000)4 ℃冰箱孵育过夜,以TBST洗膜3次,每次10 min;分别加入二抗(稀释比1∶2 000)室温下脱色摇床孵育1.5 h,以TBST洗膜3次,每次10 min;应用显影液在生物分子成像仪(LAS 500,美国GE公司)中进行曝光显影。应用Image J软件进行条带灰度值分析,结果以目的蛋白与β-actin灰度值的比值表示。
1.3 统计学处理应用SPSS 23.0统计学软件分析数据。计量资料以x±s表示,多组比较采用单因素方差分析,组间两两比较采用Bonferroni(B)检验。以P<0.05为差异有统计学意义。
2 结果 2.1 小鼠一般状况及肝组织病理学改变一般状况:正常对照组小鼠活动状态良好、行为敏捷,饮水及进食正常,皮毛光滑整洁;模型对照组小鼠活动明显减少,饮水及进食明显增加,尿量明显增多,脱毛;ISO低、中、高剂量组小鼠的一般状况与模型对照组相比有不同程度改善,以中、高剂量组改善比较明显。
油红O染色结果:正常对照组小鼠肝组织未见脂滴沉积;模型对照组小鼠肝组织可见弥漫性红色脂滴形成,数量颇多;ISO低、中、高剂量组小鼠脂滴沉积较模型对照组逐渐减少,以中、高剂量组减少较显著。见图 1。
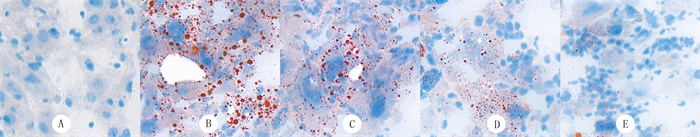 |
| A:正常对照组;B:模型对照组;C:ISO低剂量组;D:ISO中剂量组;E:ISO高剂量组。油红O染色,400倍。 图 1 各组小鼠肝组织病理学观察 |
模型对照组小鼠的血清FBG水平以及肝组织TG、TC、FFA含量较正常对照组显著增加,ISO低、中、高剂量组小鼠血清FBG水平以及肝组织TG、TC、FFA含量较模型对照组显著降低,差异均具有统计学意义(F=62.612~1 183.454,P<0.001)。且ISO作用呈剂量依赖性。见表 1。
| 表 1 各组小鼠血清FBG及肝组织脂质水平的比较(n=10,x±s) |
|
|
与正常对照组相比,模型对照组小鼠肝组织中T-AOC、SOD、CAT、GSH等抗氧化指标水平显著降低,脂质过氧化产物MDA水平显著升高;与模型对照组相比,ISO低、中、高剂量组小鼠肝组织中T-AOC、SOD、CAT、GSH水平显著升高,MDA水平则显著降低,差异均具有统计学意义(F=49.356~1 869.515,P<0.001)。且ISO作用具有明显的剂量依赖性。见表 2。
| 表 2 各组小鼠肝组织氧化应激指标比较(n=10,x±s) |
|
|
与正常对照组相比,模型对照组小鼠肝组织中Nrf-2、HO-1、GLUT2蛋白的表达水平显著降低;与模型对照组相比,ISO低、中、高剂量组小鼠肝组织中Nrf-2、HO-1及GLUT2蛋白的表达水平显著增高,差异均具有统计学意义(F=6.560~42.410,P<0.01)。ISO对Nrf-2及GLUT2蛋白表达水平的增高作用呈明显的剂量依赖性,但对HO-1蛋白表达的增高作用无剂量依赖性。见图 2、表 3。
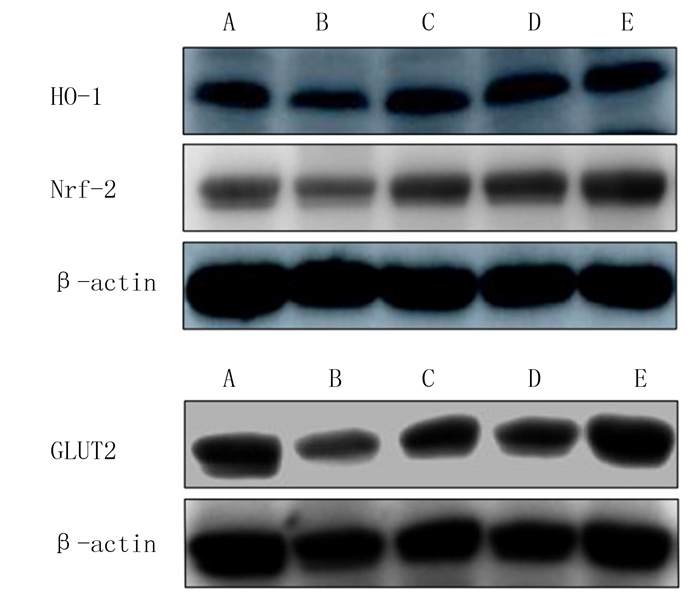 |
| A:正常对照组;B:模型对照组;C:ISO低剂量组;D:ISO中剂量组;E:ISO高剂量组。 图 2 ISO对小鼠肝组织Nrf-2、HO-1、GLUT2蛋白表达的影响 |
| 表 3 各组小鼠肝组织Nrf-2、HO-1、GLUT2蛋白表达的比较(n=5,x±s) |
|
|
T2DM病人NAFL的发病率逐年增高[1],而且T2DM和NAFL高度相关,互为因果,从而导致疾病进一步加重,严重威胁生命健康[17]。T2DM合并NAFL的发病机制尚未完全阐明。目前学术界广泛接受的学说是“二次打击”学说:首次打击是以胰岛素抵抗为主要原因导致的脂质在肝细胞胞质中的沉积;再次打击为氧化应激反应,指在首次打击的基础上,由活性氧(ROS)诱导发生在肝脏实质细胞内的一系列炎症反应[18-19]。持续的炎症反应会进一步加重胰岛素抵抗,从而促进T2DM合并NAFL的疾病进展[20-21]。因此,改善胰岛素抵抗、抗氧化是延缓T2DM合并NAFL疾病进展的重要措施。
ISO是一种黄酮类化合物,具有抗炎、抗氧化、保肝、降糖及调脂作用[12-16]。本实验通过高脂高糖饮食及腹腔注射STZ建立T2DM合并NAFL模型,同时应用ISO处理成模小鼠。结果显示,ISO处理的成模小鼠肝组织TG、TC、FFA及血清FBG水平均显著下降,肝组织脂滴沉积明显减少甚至消失,氧化应激导致的脂质过氧化产物MDA(可以间接反映体内氧自由基的水平)水平明显降低,而肝组织抗氧化体系T-AOC、SOD、CAT、GSH水平显著增高。表明ISO能改善T2DM合并NAFL模型小鼠肝脏脂质沉积,降低其FBG,抑制肝脏的氧化应激。
研究表明,NAFL是由于慢性脂质沉积导致的肝脏脂毒性炎症病变,其主要介导机制是胰岛素抵抗和氧化应激[18-19]。当机体处于氧化应激状态时其抗氧化系统被激活,Nrf-2是抗氧化体系中一种重要的核转录因子[22]。Nrf-2在肝脏中高表达,生理状态下,Nrf-2存在于肝细胞胞浆中与其分子伴侣Keap1结合处于无活性状态,当发生氧化应激时,Nrf-2和Keap1解离,Nrf-2发生核转位与抗氧化反应原件(ARE)上的GCTGAGTCA位点相结合,启动抗氧化酶基因表达(包括 HO-1 、SOD及CAT等),最终导致肝细胞抗氧化应激反应[23]。前期研究表明,Nrf-2基因缺失可加重氧化应激触发的细胞毒性[24]。本实验模型对照组氧化应激导致的脂质过氧化产物MDA水平较正常对照组明显增高,抗氧化体系T-AOC、SOD、CAT、GSH水平明显降低,说明氧化体系与抗氧化体系失衡,存在严重的氧化应激反应。而经ISO处理的模型小鼠肝组织脂质过氧化产物MDA水平较模型对照组明显降低,抗氧化体系T-AOC、SOD、CAT、GSH水平明显增高,说明ISO小鼠肝组织氧化应激反应明显减轻。同时,模型对照组小鼠肝组织抗氧化应激信号通路Nrf-2/HO-1中的相关蛋白Nrf-2、HO-1表达量较正常对照组明显降低,而经ISO处理的模型小鼠肝组织Nrf-2、HO-1蛋白的表达量较模型对照组明显增高,说明ISO通过激活Nrf-2/HO-1信号通路而起到抗氧化作用,从而减轻T2DM合并NAFL发病机制中的“第二次打击”。有研究表明,氧化应激是导致肝脏胰岛素抵抗发生的机制[25-27],因此ISO也间接减轻了T2DM合并NAFL发病机制中的“第一次打击”。
有研究表明,高脂高糖饮食除了可以诱导产生氧化应激产物MDA及ROS之外,还可导致胰岛素信号传导通路异常,诱发胰岛素抵抗[28-29]。另有研究表明,抗氧化剂可干预这一过程,改善胰岛素抵抗[30-32]。GLUT2是肝脏重要的葡萄糖转运蛋白,当机体处于高糖刺激时可促进葡萄糖自血浆进入胞质[33-35]。T2DM时肝脏葡萄糖代谢紊乱可致肝脏GLUT2水平降低,说明糖尿病大鼠肝糖原含量减少与GLUT2表达下调有关[36-37]。PI3K/Akt信号通路是经典的胰岛素信号通路,此通路激活后可促进肝脏GLUT2的转运,增加肝糖原合成,抑制肝糖原分解,降低血糖浓度,减轻T2DM病人胰岛素抵抗[36, 38]。本实验模型对照组小鼠肝组织中GLUT2蛋白的表达量显著低于正常对照组,而经ISO处理后模型小鼠肝组织中GLUT2蛋白的表达量显著提高。GLUT2蛋白表达量提高可以促进肝细胞对葡萄糖摄取,增加肝糖原合成,进一步缓解T2DM合并NAFL小鼠肝脏胰岛素抵抗,从而降低血糖水平以及减少以胰岛素抵抗为中心的肝脏脂质沉积,改善NAFL病情。
综上所述,ISO能够减轻T2DM合并NAFL小鼠肝脏的氧化应激、胰岛素抵抗及降低血糖,从而延缓T2DM合并NAFLD进展、减轻病情。其机制与激活Nrf-2/HO-1信号通路,增加肝脏GLUT2蛋白表达有关。本研究为ISO作为一种治疗T2DM合并NAFL的新药提供了实验依据。但ISO改善T2DM合并NAFL小鼠肝脏脂质沉积的具体分子机制尚不清楚,有待进一步深究。
| [1] |
SMITH B W, ADAMS L A. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment[J]. Nature Reviews Endocrinology, 2011, 7(8): 456-465. DOI:10.1038/nrendo.2011.72 |
| [2] |
YATSUJI S, HASHIMOTO E, KANEDA H, et al. Diagnosing autoimmune hepatitis in nonalcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful[J]. Journal of Gastroenterology, 2005, 40(12): 1130-1138. DOI:10.1007/s00535-005-1711-z |
| [3] |
AYGUN C, KOCAMAN O, SAHIN T, et al. Evaluation of metabolic syndrome frequency and carotid artery intima-media thickness as risk factors for atherosclerosis in patients with nonalcoholic fatty liver disease[J]. Digestive Diseases and Sciences, 2008, 53(5): 1352-1357. DOI:10.1007/s10620-007-9998-7 |
| [4] |
GONG J Y, XIA D Z, HUANG J, et al. Functional components of bamboo shavings and bamboo leaf extracts and their antioxidant activities in vitro[J]. Journal of Medicinal Food, 2015, 18(4): 453-459. DOI:10.1089/jmf.2014.3189 |
| [5] |
SOULIMANI R, YOUNOS C, JARMOUNI S, et al. Beha-vioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse[J]. Journal of Ethnopharmacology, 1997, 57(1): 11-20. DOI:10.1016/S0378-8741(97)00042-1 |
| [6] |
SEZIK E, ASLAN M, YESILADA E, et al. Hypoglycaemic activity of Gentiana olivieri and isolation of the active consti-tuent through bioassay-directed fractionation techniques[J]. Life Sciences, 2005, 76(11): 1223-1238. DOI:10.1016/j.lfs.2004.07.024 |
| [7] |
MALI V R, MOHAN V, BODHANKAR S L. Antihyperten-sive and cardioprotective effects of the Lagenaria siceraria fruit in NG-nitro-L-arginine methyl ester (L-NAME) induced hypertensive rats[J]. Pharmaceutical Biology, 2012, 50(11): 1428-1435. DOI:10.3109/13880209.2012.684064 |
| [8] |
LIN X, CHEN Y X, LV S, et al. Gypsophila elegans isoorientin attenuates CCl4-induced hepatic fibrosis in rats via modulation of NF-κB and TGF-β1/Smad signaling pathways[J]. International Immunopharmacology, 2015, 28(1): 305-312. DOI:10.1016/j.intimp.2015.06.021 |
| [9] |
REVILLA-MONSALVE M C, ANDRADE-CETTO A, PA-LOMINO-GARIBAY M A, et al. Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extracts on type 2 diabetic patients[J]. Journal of Ethnopharmacology, 2007, 111(3): 636-640. DOI:10.1016/j.jep.2007.01.014 |
| [10] |
GUNDOGDU G, DODURGA Y, ELMAS L, et al. Investigation of the anticancer mechanism of isoorientin isolated from Eremurus spectabilis leaves via cell cycle pathways in HT-29 human colorectal adenocarcinoma cells[J]. The Eurasian Journal of Medicine, 2018, 50(3): 168-172. DOI:10.5152/eurasianjmed.2018.17403 |
| [11] |
KIM S J, ZAIDUL I S, SUZUKI T, et al. Comparison of phenolic compositions between common and Tartary buckwheat (Fagopyrum) sprouts[J]. Food Chemistry, 2008, 110(4): 814-820. DOI:10.1016/j.foodchem.2008.02.050 |
| [12] |
YUAN L, WU Y C, REN X M, et al. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia[J]. Molecular and Cellular Biochemistry, 2014, 386(1-2): 153-165. DOI:10.1007/s11010-013-1854-9 |
| [13] |
CHOI J S, ISLAM M N, ALI M Y, et al. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer's disease, anti-diabetic, and anti-inflammatory activities[J]. Archives of Pharmacal Research, 2014, 37(10): 1354-1363. DOI:10.1007/s12272-014-0351-3 |
| [14] |
HUANG Q F, ZHANG S J, ZHENG L, et al. Protective effect of isoorientin-2″-O-α-l-arabinopyranosyl isolated from Gypsophila elegans on alcohol induced hepatic fibrosis in rats[J]. Food and Chemical Toxicology, 2012, 50(6): 1992-2001. DOI:10.1016/j.fct.2012.03.044 |
| [15] |
PARK H S, LIM J H, KIM H J, et al. Antioxidant flavone glycosides from the leaves of Sasa borealis[J]. Archives of Pharmacal Research, 2007, 30(2): 161-166. DOI:10.1007/BF02977689 |
| [16] |
ALONSO-CASTRO A J, ZAPATA-BUSTOS R, GÓMEZ-ESPINOZA G, et al. Isoorientin reverts TNF-α-induced insulin resistance in adipocytes activating the insulin signaling pathway[J]. Endocrinology, 2012, 153(11): 5222-5230. DOI:10.1210/en.2012-1290 |
| [17] |
LEE B W, LEE Y H, PARK C Y, et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research group of the Korean diabetes association[J]. Diabetes & Metabolism Journal, 2020, 44(3): 382-401. |
| [18] |
CHEN Z W, CHEN L Y, DAI H L, et al. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease[J]. Journal of Zhejiang University Science B, 2008, 9(8): 616-622. DOI:10.1631/jzus.B0720016 |
| [19] |
JAMES O F, DAY C P. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance[J]. Journal of Hepatology, 1998, 29(3): 495-501. DOI:10.1016/S0168-8278(98)80073-1 |
| [20] |
BUZZETTI E, PINZANI M, TSOCHATZIS E A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)[J]. Metabolism: Clinical and Experimental, 2016, 65(8): 1038-1048. DOI:10.1016/j.metabol.2015.12.012 |
| [21] |
KIM J K, KIM Y J, FILLMORE J J, et al. Prevention of fat-induced insulin resistance by salicylate[J]. The Journal of Cli-nical Investigation, 2001, 108(3): 437-446. DOI:10.1172/JCI11559 |
| [22] |
KLAASSEN C D, REISMAN S A. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver[J]. Toxicology and Applied Pharmacology, 2010, 244(1): 57-65. DOI:10.1016/j.taap.2010.01.013 |
| [23] |
WANG X, TAO L, HAI C X. Redox-regulating role of insulin: the essence of insulin effect[J]. Molecular and Cellular Endocrinology, 2012, 349(2): 111-127. DOI:10.1016/j.mce.2011.08.019 |
| [24] |
COPPLE I M, GOLDRING C E, KITTERINGHAM N R, et al. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity[J]. Toxicology, 2008, 246(1): 24-33. |
| [25] |
MAIESE K, MORHAN S D, CHONG Z Z. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus[J]. Current Neurovascular Research, 2007, 4(1): 63-71. DOI:10.2174/156720207779940653 |
| [26] |
PETERSEN M C, SHULMAN G I. Mechanisms of insulin action and insulin resistance[J]. Physiological Reviews, 2018, 98(4): 2133-2223. DOI:10.1152/physrev.00063.2017 |
| [27] |
RIVES C, FOUGERAT A, ELLERO-SIMATOS S, et al. Oxidative stress in NAFLD: role of nutrients and food contaminants[J]. Biomolecules, 2020, 10(12): E1702. DOI:10.3390/biom10121702 |
| [28] |
CIRCU M L, AW T Y. Reactive oxygen species, cellularredox systems, and apoptosis[J]. Free Radical Biology andMedicine, 2010, 48(6): 749-762. DOI:10.1016/j.freeradbiomed.2009.12.022 |
| [29] |
NAKAMURA S, TAKAMURA T, MATSUZAWA-NAGATA N, et al. Palmitate induces insulin resistance in H4ⅡEC3 hepatocytes through reactive oxygen species produced by mitochondria[J]. The Journal of Biological Chemistry, 2009, 284(22): 14809-14818. DOI:10.1074/jbc.M901488200 |
| [30] |
WANG X, GU C S, HE W, et al. Glucose oxidase induces insulin resistance via influencing multiple targets in vitro and in vivo: The central role of oxidative stress[J]. Biochimie, 2012, 94(8): 1705-1717. DOI:10.1016/j.biochi.2012.03.024 |
| [31] |
STRAUB L G, EFTHYMIOU V, GRANDL G, et al. Antioxidants protect against diabetes by improving glucose homeostasis in mouse models of inducible insulin resistance and obesity[J]. Diabetologia, 2019, 62(11): 2094-2105. |
| [32] |
VAN DER SCHAFT N, SCHOUFOUR J D, NANO J, et al. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: the Rotterdam Study[J]. European Journal of Epidemiology, 2019, 34(9): 853-861. DOI:10.1007/s10654-019-00548-9 |
| [33] |
THORENS B. GLUT2, glucose sensing and glucose homeostasis[J]. Diabetologia, 2015, 58(2): 221-232. DOI:10.1007/s00125-014-3451-1 |
| [34] |
SCHMIDL S, URSU O, IANCU C V, et al. Identification of new GLUT2-selective inhibitors through in silico ligand screening and validation in eukaryotic expression systems[J]. Scientific Reports, 2021, 11(1): 13751. |
| [35] |
BERGER C, ZDZIEBLO D. Glucose transporters in pancreatic islets[J]. Pflugers Archiv, 2020, 472(9): 1249-1272. |
| [36] |
REN Z Q, ZHANG P B, ZHANG X Z, et al. Duodenal-jejunal exclusion improves insulin resistance in type 2 diabetic rats by upregulating the hepatic insulin signaling pathway[J]. Nutrition (Burbank, Los Angeles County, Calif), 2015, 31(5): 733-739. DOI:10.1016/j.nut.2014.10.012 |
| [37] |
KHALID M, ALKAABI J, KHAN M A B, et al. Insulin signal transduction perturbations in insulin resistance[J]. International Journal of Molecular Sciences, 2021, 22(16): 8590. |
| [38] |
WANG K P, TANG Z H, ZHENG Z M, et al. Protective effects of Angelica sinensis polysaccharide against hyperglycemia and liver injury in multiple low-dose streptozotocin-induced type 2 diabetic BALB/c mice[J]. Food & Function, 2016, 7(12): 4889-4897. |
 2022, Vol. 58
2022, Vol. 58

