2. 山东医学高等专科学校儿科学教研室
文献报道,线粒体脑肌病伴乳酸血症和卒中样发作(MELAS)是由于线粒体DNA发生突变,导致线粒体结构和功能异常,引起细胞能量代谢障碍[1],常累及多个器官,以中枢神经系统、骨骼肌和心肌等高需能部位最为常见,是线粒体脑肌病中最为常见的一种类型,为母系遗传病。MELAS临床表现复杂且缺乏特异性,常被误诊为癫痫、脑炎、脑梗死及脱髓鞘脑病等而延误有效治疗,导致预后较差[2]。虽然近年来国内有关MELAS的研究报道不断增多,但MELAS早期的误诊率仍极高,所以探讨如何早期诊断及治疗就显得尤为重要。本研究对7例MELAS病儿的临床特点、实验室检查、神经电生理、影像学表现及基因检测结果等进行了回顾性分析,并探讨儿童MELAS线粒体基因A3243G点突变异质性水平与临床特征的关系,以期为MELAS的早期临床诊断及临床遗传咨询提供依据。
1 资料与方法 1.1 研究对象研究对象为我院小儿神经内科2011年1月—2018年12月收治的7例MELAS病儿,男5例,女2例,年龄3~10岁。
1.2 研究方法回顾性分析MELAS病儿的首发症状、起病年龄、治疗过程、生长发育史、既往疾病史、家族史、体型、皮肤毛发改变、智力倒退程度、血乳酸水平、颅脑影像学特征(部位、信号强度特点等)、脑电图表现(背景活动、痫样放电与影像学的对应关系)等资料。取所有研究对象外周静脉血2 mL,对线粒体基因进行二代测序及多重连接探针扩增技术(MLPA)检测,观察线粒体基因A3243G点突变情况,并分析该基因位点突变率与起病年龄、血乳酸水平的相关性。采用SPSS 16.0统计软件进行数据处理,所得计量数据以x±s表示,相关性分析采用Pearson积矩相关系数法,以P < 0.05为差异有统计学意义。
2 结果 2.1 临床特点本文7例病儿起病年龄为3~10岁,平均(7.42±2.57)岁。7例中6例以抽搐为首发症状,1例以头痛、呕吐起病;伴体型瘦小者7例,多毛6例,发热4例,视力障碍3例,肌病表现2例,智力运动发育落后1例,房间隔缺损1例。误诊为癫痫3例,病毒性脑炎2例。1例在随访过程中死亡。
2.2 实验室检查本文7例病儿血乳酸水平均升高,平均为(7.87±2.48)mmol/L(正常参考值为0.5~2.0 mmol/L)。7例病儿颅脑MRI示顶、枕、颞叶异常信号,不符合解剖学血管支配分布,见图 1。脑电图特点:7例病儿均有背景活动慢化,1例局灶性发作起始部位与颅脑影像学病变部位一致。
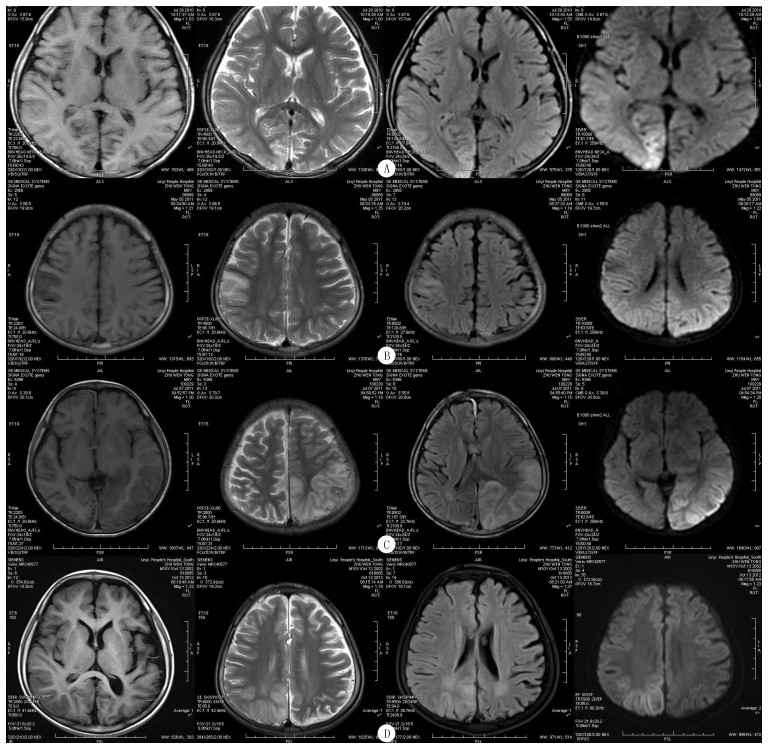 |
| A:首诊时MRI平扫示右侧枕叶沿脑回走行异常信号,DWI表现较其他序列明显,呈稍高信号,T1WI呈稍低信号,T2WI及T2压水序列呈稍高信号。B:2011年5月5日(8个月后)检查,右侧顶叶主要在中央后回出现新病灶,原右枕叶病灶区呈萎缩性改变,局部见胶质增生。C:2011年7月7日检查,左侧颞、顶、枕叶出现大片状病灶,以皮质及皮质下白质受累为主,三角区白质相对保持完好,脑回肿胀,DWI呈高信号,ADC图呈高信号,提示为血管源性水肿。D:2012年10月13日检查示右顶叶新发病灶,DWI呈稍高信号,病变区脑回肿胀,脑沟变窄;原左侧颞、顶、枕叶病变区呈脑萎缩性改变,局部脑沟增宽,但脑实质信号改变不明显。 图 1 1例病儿不同时期MRI特点 |
本文7例病儿的致病基因均为MT-TL1基因A3243G突变,其突变率为(43±14)%;7例病儿母亲及1例病儿姐姐无临床表现,其突变率为10%~20%;病儿父亲均未发现突变。其中2例病儿为双胞胎兄弟,先证者发病后即刻对其胞弟进行基因检测,结果显示A3243G突变率>30%,其弟1年后发病;1例病儿及其母亲、姐姐线粒体MLPA还同时检测到MTND2杂合缺失变异。见图 2。
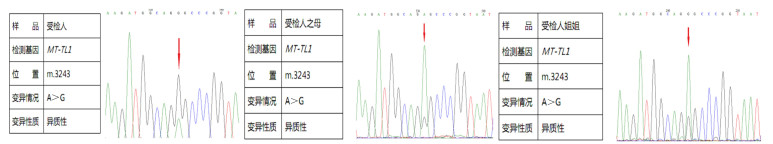 |
| 图 2 1例病儿及其母亲、姐姐基因检测结果 |
MELAS病儿线粒体基因A3243G突变率与血乳酸水平呈正相关(r=0.89,P < 0.01),与起病年龄呈负相关(r=-0.84, P < 0.05)。
3 讨论人类线粒体DNA是由16 569个碱基对组成的双链环状分子,含37个基因,主要编码呼吸链和与能量代谢有关的蛋白,其结构和功能异常往往导致整个能量代谢过程紊乱,从而产生一系列的疾病。线粒体脑肌病是一组少见的线粒体结构和(或)功能异常所导致的以脑和肌肉受累为主的多系统疾病。MELAS是线粒体脑肌病最常见的一种类型,具有高度临床变异性和遗传异质性,是一种以卒中样发作和乳酸酸中毒为特征的母系遗传病。MELAS发病年龄为3~40岁,以5~15岁起病最为常见。本组病儿起病年龄3~10岁, 平均7.7岁。MELAS临床可表现为卒中样发作[3]、疲乏无力、身材矮小、多毛、头痛和呕吐、视觉障碍、偏瘫、失语、癫痫、心律失常、胃肠功能紊乱、肾脏损害、精神行为异常、内分泌障碍、认知障碍等[4-12],重症可致死亡[13-15],临床表现复杂多样,所以误诊率极高。本组7例病儿中6例以抽搐为首发症状,1例以头痛、呕吐起病;伴体型瘦小7例,多毛6例,发热4例,视力障碍3例,肌病表现2例,智力运动发育落后1例,房间隔缺损1例。由于临床表现复杂,本组误诊5例,其中误诊为癫痫3例,误诊为病毒性脑炎2例。本组57%的病儿出现发热,发热是误诊为病毒性脑炎的原因之一。发热时代谢增高,ATP消耗增多,加重了线粒体的负担,加之氧化应激产生过多的氧自由基,可导致线粒体衰竭而死亡,从而出现一系列的临床症状。因此,感染发热是MELAS的一个重要诱因。尤其需要注意的是,本组以抽搐为首发症状者比例明显高于以往文献报道[7]。反复出现的抽搐发作且发作间期未见脑电图的异常,则使MELAS易被误诊为特发性癫痫。
MELAS的脑内病灶可单发或散在多发,多呈不对称分布,双侧脑叶发病概率基本相同,本组病例发病区域主要位于顶、枕叶,其次为颞叶。MELAS可反复发作,本组同一病儿的多次MRI检查可见病灶具有游走性、多变性,这是其有别于其他疾病的另一重要影像学特点[16-18]。综合该病儿多次MRI检查来看,MELAS脑MRI表现具有一定的特点:①病灶脑回状分布,以累及皮质及皮质下白质为主,当然也有只累及深部灰质核团的报道,但本组病例未见;②病灶不按脑血管支配区分布,以累及颞、顶、枕叶为主,额叶少见;③病灶具有游走性,此起彼伏,反复发作,最终趋向于文献所报道的对称性分布(这种对称不是一次形成的);④血管源性水肿为主,DWI表现为高信号,ADC图呈高信号,可与脑梗死鉴别。功能MRI对MELAS鉴别至关重要。本组7例脑电图检查示发作间期均以背景活动慢化为主,未见痫样放电,1例部分性发作起始部位与颅脑影像学病变部位有高度相关性,基因检查示mtDNA3243位点突变,符合文献报道的MELAS表现。
基因突变分析是确诊MELAS的重要手段之一。MELAS以mtDNA突变为主,其突变位点很多,可位于tRNA、COXⅢ、ND3和ND5[19-21]。其中发现最早、最常见突变是编码tRNA的mtDNA A3243G突变[13],占80%左右。本组7例病儿经基因突变分析证实均为mtDNA A3243G突变,此突变是我国儿童MELAS最常见的突变。而1例病儿及其母亲、姐姐在检测到mtDNA A3243G突变的同时还于MLPA检测到MTND2杂合缺失突变,此变异尚未见到文献报道与MELAS相关。ND2基因是线粒体DNA中的一个蛋白编码基因,其编码蛋白是构成线粒体呼吸链复合物中的重要亚单位之一,并且参与线粒体呼吸链和氧化磷酸化功能。此例病儿临床症状重、起病年龄早不能确定是否与合并ND2杂合缺失突变有关。线粒体脑肌病是母系遗传病,子代遗传的突变mtDNA占总mtDNA的比例较其上一代有所增加,异质性和阈效应导致同一母亲的不同子女临床表型亦有很多的不同。只有当突变的DNA占一定比例时才会引起临床症状,这个比例被称为阈值,而这种现象则被称为阈效应。突变的异质性及器官对突变敏感性程度的不同,可导致不同缺陷的临床症状[22]。同时,A3243G突变存在很大的临床异质性,使得携带同一个突变的个体呈现不同类型、不同程度的临床表型,有些表型甚至终身不发病。血清与脑脊液乳酸水平升高是诊断MELAS重要的生化指标[23],乳酸水平与神经损伤的严重程度相关。本组7例病儿血乳酸水平均大于5 mmol/L,其中病情较重的1例病儿血乳酸水平最高。本文结果显示,MELAS病儿A3243G突变率与血乳酸水平呈正相关,与起病年龄呈负相关,表明MELAS病儿A3243G突变率高低可提示临床症状轻重程度。目前确诊MELAS的金标准是在病理、基因和生化检查中发现线粒体有结构和功能异常,单纯测定乳酸缺乏特异性。影像学检查虽然有特征性表现,但其出现较晚。骨骼肌活检因其有创性、局限性,症状较轻的病人多不能配合。随着二代基因测序技术的蓬勃发展,基因测序速度极大提升、成本大幅下降,为MELAS早期诊断开辟了新的途径。
本病目前尚无特殊治疗方法,除传统的“鸡尾酒”疗法外,有研究表明,天然奎宁化合物β-拉帕醌可减轻线粒体功能障碍[24];另有报道可通过诱导多能干细胞进行自体细胞替代治疗[25];还有报道生酮饮食可通过改善线粒体功能而控制抽搐及卒中样发作[26];也有学者认为,通过对瓜氨酸及精氨酸的补充从而增加一氧化氮的生成,减轻脑血流灌注受损,对MELAS可能起到治疗作用[27]。但最终疗效目前尚无大样本临床报道。由此看来,遗传性筛查尤为重要,对病人及其家庭成员进行婚姻及生育方面指导,能防患于未然。同时本病需做好家系调查,筛查基因携带者,并进行必要的遗传学指导。
综上所述,儿童MELAS临床表现复杂多样,首发症状不一,早期诊断困难,需注意与病毒性脑炎、脑血管病等相鉴别。身材矮小、多毛为其常见体征,对于合并多种不相关的器官受累者要高度警惕该病,当临床遇有表型典型和(或)具有特征性影像学改变者应想到MELAS,可行血乳酸、脑电图检查进行初筛,可疑者进一步行基因突变检测。充分认识MELAS的临床、生化、颅脑影像、病理、基因突变及预后特点,将有助于其早期诊断与治疗,并有助于遗传咨询。早期筛查家系以及进行基因检测有助于MELAS的诊断,病儿线粒体基因A3243G突变率的高低可能为该病临床严重程度及预后评估提供依据,但这仍需大样本研究进一步证实。
| [1] |
LIN D S, HUANG Y W, HO C S, et al. Oxidative insults and mitochondrial DNA mutation promote enhanced autophagy and mitophagy compromising cell viability in pluripotent cell model of mitochondrial disease[J]. Cells, 2019, 8(1): 65. |
| [2] |
SUN Xiangrong, JIANG Guohui, JU Xinyue, et al. MELAS and macroangiopathy: a case report and literature review[J]. Medicine, 2018, 97(52): e13866. DOI:10.1097/MD.0000000000013866 |
| [3] |
FRYER R H, BAIN J M, DE VIVO D C. Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS): a case report and critical reappraisal of treatment options[J]. Pediatric Neurology, 2016, 56: 59-61. DOI:10.1016/j.pediatrneurol.2015.12.010 |
| [4] |
LEE S, OH D A, BAE E K. Fixation-off sensitivity in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome[J]. Seizure, 2019, 64: 6-7. DOI:10.1016/j.seizure.2018.11.010 |
| [5] |
THOMAS T, CRAIGEN W J, MOORE R, et al. Arrhythmia as a cardiac manifestation in MELAS syndrome[J]. Mol Genet Metab Rep, 2015, 4: 9-10. DOI:10.1016/j.ymgmr.2015.05.002 |
| [6] |
GE Yuxing, SHANG Bo, CHEN Wenzhen, et al. Adult-onset of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome with hypothyroidism and psychiatric disorders[J]. eNeurologicalSci, 2016, 6: 16-20. |
| [7] |
EL-HATTAB A W, ADESINA A M, JONES J A. MELAS syndrome:clinical manifestations, pathogenesis, and treatment options[J]. Molecular Genetics and Metabolism, 2015, 116(1/2, SI): 4-12. |
| [8] |
FINSTERER J, ZARROUK-MAHJOUB S. Gastrointestinal involvement in m.3243A > G-associated MELAS[J]. Internal Medicine, 2018, 57(5): 769-770. DOI:10.2169/internalmedicine.9439-17 |
| [9] |
SUZUKI J, IWATA M, MORIYOSHI H, et al. Gastro-intestinal involvement in m. m.3243A > G-associated mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes[J]. Internal Medicine, 2018, 57(5): 771. DOI:10.2169/internalmedicine.9632-17 |
| [10] |
KRAYA T, DESCHAUER M, JOSHI P R, et al. Prevalence of headache in patients with mitochondrial disease: a cross-sectional study[J]. Headache, 2018, 58(1): 45-52. DOI:10.1111/head.13219 |
| [11] |
VOLLONO C, PRIMIANO G, DELLA MARCA G A, et al. Migraine in mitochondrial disorders:prevalence and characte-ristics[J]. Cephalalgia: an International Journal of Headache, 2018, 38(6): 1093-1106. DOI:10.1177/0333102417723568 |
| [12] |
FINSTERER J, FRANK M. Renal involvement in MELAS[J]. Medicina Clinica, 2017, 149(7): 314-321. DOI:10.1016/j.medcli.2017.03.053 |
| [13] |
LANGDAHL J H, LARSEN M, FROST M, et al. Lecocytes mutation load declines with age in carriers of the m.3243A > G mutation: a 10-year prospective cohort[J]. Clinical Genetics, 2018, 93(4): 925-928. DOI:10.1111/cge.13201 |
| [14] |
KONDO H, FUJITA Y, MIZUNO Y, et al. Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes with severe systemic symptoms:pathology and biochemistry[J]. Pediatrics International, 2018, 60(3): 300-302. DOI:10.1111/ped.13472 |
| [15] |
ZHANG Zhe, ZHAO Danhua, ZHANG Xiao, et al. Survival analysis of a cohort of Chinese patients with mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) based on clinical features[J]. Journal of the Neurological Sciences, 2018, 385: 151-155. DOI:10.1016/j.jns.2017.12.033 |
| [16] |
BINDU P S, SONAM K, GOVINDARAJ P, et al. Outcome of epilepsy in patients with mitochondrial disorders:phenotype genotype and magnetic resonance imaging correlations[J]. Clinical Neurology and Neurosurgery, 2018, 164: 182-189. DOI:10.1016/j.clineuro.2017.12.010 |
| [17] |
HENRY C, PATEL N, SHAFFER W, et al. Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes-MELAS syndrome[J]. The Ochsner Journal, 2017, 17(3): 296-301. |
| [18] |
WHITEHEAD M T, WIEN M, LEE B, et al. Black toenail sign in MELAS syndrome[J]. Pediatric Neurology, 2017, 75: 61-65. DOI:10.1016/j.pediatrneurol.2017.06.017 |
| [19] |
LIM B C, PARK J D, HWANG H, et al. Mutations in ND subunits of complex Ⅰ are an important genetic cause of childhood mitochondrial encephalopathies[J]. Journal of Child Neurology, 2009, 24(7): 828-832. DOI:10.1177/0883073808331085 |
| [20] |
MUKAI M, NAGATA E. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) due to a m.10158T > C ND3 mutation with a normal muscle biopsy[J]. Internal Medicine, 2017, 56(19): 2695. DOI:10.2169/internalmedicine.8962-17 |
| [21] |
TOYOSHIMA Y, TANAKA Y, YASUDA S, et al. MELAS syndrome associated with a new mitochondrial tRNA-Val gene mutation (m.1616A > G)[J]. Annals of Neurology, 2017, 76(18, SI): S67-S68. DOI:10.1136/bcr-2017-220934 |
| [22] |
MESEGUER S, PANADERO J, NAVARRO-GONZALEZ C, et al. The MELAS mutation m.3243A > G promotes reactivation of fetal cardiac genes and an epithelial-mesenchymal transition-like program via dysregulation of miRNAs[J]. Biochimica et Biophysica Acta-Molecular Basis of Disease, 2018, 1864(9, B): 3022-3037. DOI:10.1016/j.bbadis.2018.06.014 |
| [23] |
GOODFELLOW J A, DANI K, STEWART W, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes: an important cause of stroke in young people[J]. Postgraduate Medical Journal, 2012, 88(140): 326-334. |
| [24] |
JEONG M H, KIM J H, SEO K S, et al. β-Lapachone at te-nuates mitochondrial dysfunction in MELAS cybrid cells[J]. Biochemical and Biophysical Research Communications, 2014, 454(3): 417-422. DOI:10.1016/j.bbrc.2014.10.093 |
| [25] |
KODAIRA M, HATAKEYAMA H, YUASA S, et al. Impaired respiratory function in MELAS-induced pluripotent stem cells with high heteroplasmy levels[J]. FEBS Open Bio, 2015, 5: 219-225. DOI:10.1016/j.fob.2015.03.008 |
| [26] |
STERIADE C, ANDRADE D M, FAGHFOURY H A, et al. Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) may respond to adjunctive ketogenic diet[J]. Pediatric Neurology, 2014, 50(5): 498-502. DOI:10.1016/j.pediatrneurol.2014.01.009 |
| [27] |
EL-HATTAB AW, ALMANNAI M, SCAGLIA F. Arginine and citrulline for the treatment of MELAS syndrome[J]. J Inborn Errors Metab Screen, 2017, 5: 1-5. |
 2020, Vol. 56
2020, Vol. 56

