复发性流产(RSA)的发病率占育龄期妇女的1%~5%[1-2],其发病机制主要与女方自身免疫应答有关,如果其对带有男方抗原的胚胎产生免疫攻击,就会造成母体的流产[3-4]。免疫学研究表明,机体的免疫应答主要是通过CD4+T细胞介导的细胞免疫进行,而Treg可以抑制CD4+T细胞活性,维持机体的免疫平衡[5]; 同时,白细胞介素-10(IL-10)可以增加Treg活性[6],两者在机体的免疫调节方面有很重要的作用。但两者是否对RSA有预测价值尚不明确。本文对108例妊娠早期孕妇进行前瞻性研究,探讨外周血CD4+CD25+ Treg细胞和IL-10水平对RSA的预测价值,现将结果报告如下。
1 资料与方法 1.1 一般资料2017年1月—2018年1月,选取在我院就诊的妊娠早期孕妇。纳入标准:①妊娠期在10周以内; ②年龄28~35岁; ③均行外周血染色体检查。排除标准:①合并有生殖系统感染; ②合并生殖系统解剖结构异常; ③合并妇科系统肿瘤; ④合并心血管、脑、造血系统、肾等重要脏器疾病。共纳入108例孕妇,根据既往病史是否存在RSA分为RSA组(50例)和非RSA组(对照组,58例),两组孕妇一般资料比较差异无统计学意义(P>0.05)。见表 1。本研究充分告知病人并经我院伦理委员会审批。
| 表 1 两组孕妇一般资料比较(x±s) |
|
|
采集两组孕妇空腹静脉血,EDTA-K2抗凝,颠倒混匀,送检验科进行检测。
1.2.1 外周血Treg细胞检测分离外周血单个核细胞制成细胞悬液,加入CD4和CD25抗体,避光反应15 min,加入破膜剂破膜,反应30 min后,加入PBS洗涤。离心机离心5 min,去上清,加入10 μL的APC大鼠同型对照抗体。反应30 min后,加入PBS洗涤,使用流式细胞仪测定淋巴细胞亚型。
1.2.2 外周血IL-10水平的检测将外周血高速离心后,取上层血清,应用ELISA法测定IL-10水平,所用ELISA试剂盒购自北京义翘神州生物科技有限公司。
1.3 统计学处理采用SPSS 23.0统计软件进行数据处理,计量资料结果采用x±s表示,数据间比较采用t检验; 计数资料采用百分率表示,组间比较采用χ2检验。采用受试者工作特征(ROC)曲线评价预测效能,计算曲线下面积(AUC),并应用Youden指数寻找最佳截点。当P<0.05时为差异有统计学意义。
2 结果 2.1 两组外周血CD4+CD25+Treg细胞和IL-10水平比较RSA组CD4+CD25+Treg细胞和IL-10水平均显著低于对照组,差异有显著意义(t=-8.034、-10.929,P<0.01)。见表 2。
| 表 2 两组外周血CD4+CD25+Treg细胞和IL-10水平比较(x±s) |
|
|
ROC曲线显示,CD4+CD25+Treg细胞和IL-10预测RSA具有较高的诊断效能,其AUC值分别为0.856和0.852,差异无统计学意义(P>0.05)。CD4+CD25+Treg细胞和IL-10联合检测预测RSA的效能显著高于各项指标的单独预测(Z=2.557、2.818,P<0.05);Youden指数显示,CD4+CD25+Treg细胞、IL-10水平和联合预测RSA的最佳截点分别为≤6.412%,≤15.118 μg/L和≥0.344。见图 1、表 3。
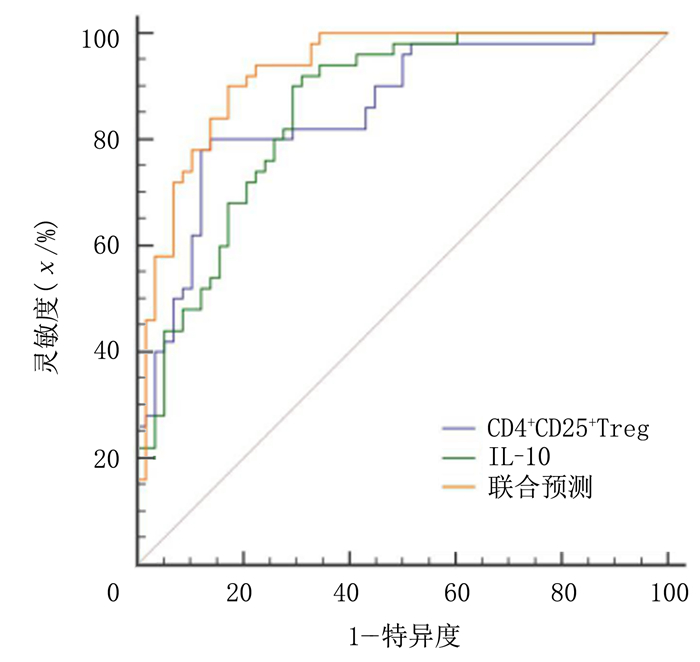 |
| 图 1 外周血CD4+CD25+Treg细胞和IL-10预测RSA的ROC曲线 |
| 表 3 外周血CD4+CD25+Treg细胞和IL-10水平预测RSA的AUC |
|
|
外周血CD4+CD25+Treg细胞和IL-10联合检测的灵敏度为94.00%,显著高于各项指标单独预测的灵敏度(χ2=5.316、4.332,P<0.05)。见表 4。
| 表 4 外周血CD4+CD25+Treg细胞和IL-10检测对RSA的预测效能(χ/%) |
|
|
低水平CD4+CD25+Treg细胞+IL-10的RSA孕妇、高水平CD4+CD25+Treg细胞+IL-10的RSA孕妇本次怀孕自然流产的发生率分别为28%和12%,低水平CD4+CD25+Treg细胞+IL-10的RSA孕妇再发自然流产的风险明显高于高水平CD4+CD25+Treg细胞+IL-10的RSA孕妇,差异有统计学意义(HR=2.611, 95%CI=1.085~6.283, P<0.05)。见图 2。
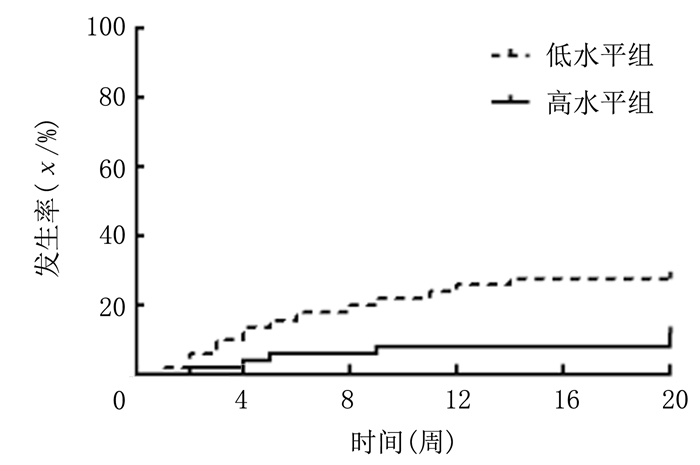 |
| 图 2 不同外周血CD4+CD25+Treg细胞和IL-10水平孕妇本次怀孕自然流产的发生情况 |
正常妊娠依赖于母体和胎儿之间的免疫平衡,当母体对胎儿的免疫攻击增加,就会导致不同类型的流产[7]。RSA在临床上是一种复杂的病症,给病人及其家庭造成了巨大的精神压力[8]。大量研究表明,不明原因的RSA主要与孕妇免疫应答混乱、自身免疫过于亢进、免疫耐受下降相关[9],该类孕妇怀孕成功与否取决于母体对胚胎中携带父方的基因是否有足够的免疫耐受[10]。目前,越来越多的RSA研究集中在CD4+T细胞上,研究发现,RSA病人外周血中的CD4+CD25+Treg细胞水平较低,CD4+CD25+Treg细胞与母体的免疫排斥有着密切的联系[11]; 而IL-10对Treg的活性有着很强的激活作用。因此,RSA可能与CD4+CD25+Treg细胞以及IL-10有关[12-13]。如果能在孕早期找到预测RSA的临床免疫指标,可以为临床早期诊断RSA并尽早采取预防措施提供参考,从而降低流产率,提高怀孕成功率。
CD4+CD25+Treg细胞是CD4+T细胞中的一个亚型,该类细胞通过产生细胞毒性作用,导致免疫损伤,同时,CD4+CD25+Treg细胞可以抑制IL-2基因在CD4+和CD8+细胞中的转录和翻译,从而抑制CD4+和CD8+两类细胞的增殖和活性。因此, 其主要作用为维持机体的免疫耐受,对全身免疫功能的正常运行起着重要的作用[14-16]。IL-10对CD4+CD25+Treg细胞活性的增强以及转化起着重要的作用[17],大量的研究结果表明,妇女孕期中IL-10的水平会升高,CD4+CD25+Treg细胞表面的IL-10受体增多,使CD4+CD25+Treg细胞更易被IL-10所活化; 同时,CD4+CD25+Treg细胞会进一步分泌IL-10[18-20]。IL-10可以使Th17细胞转化为CD4+CD25+Treg细胞,增加其在外周血中的细胞密度[21]。可见,IL-10和CD4+CD25+Treg细胞两者互相促进,协同作用,共同维持机体的免疫稳定。
IL-10和CD4+CD25+Treg细胞水平越高,其对CD4+T细胞免疫功能的抑制就越强,母体对胚胎的免疫攻击越弱,从而使母体和胎儿达到一定程度的免疫稳态[22-24]。在本研究中,RSA组CD4+CD25+Treg细胞和IL-10水平显著低于对照组,提示低水平CD4+CD25+Treg细胞+IL-10与RSA有一定关系,这与相关研究结果一致[25]。RSA病人常存在染色体异常,导致IL-10及CD4+CD25+Treg细胞水平降低,机体免疫功能紊乱,对CD4+T细胞免疫功能的抑制强度下降,使机体的自身免疫功能增加[26]。而胚胎作为一种携带有父方基因的外来组织,首当其冲受到母体的免疫攻击,造成流产[27]。同时,免疫记忆功能使机体对胚胎的免疫攻击效应持续存在,导致孕妇出现反复流产[28]。
此外,本文ROC曲线分析显示,CD4+CD25+Treg细胞和IL-10对RSA有较高的预测效能,且联合检测对RSA的预测效能及灵敏度均显著高于两项指标单独预测,提示CD4+CD25+Treg细胞和IL-10对RSA有着较好的预测价值,联合检测能提高RSA的预测能力,这与国外相关学者研究结果一致[29]。CD4+CD25+Treg细胞和IL-10在维持机体的免疫耐受过程中发挥协同作用[30],因此两种指标联合检测对预测RSA有重要意义。本研究的意义在于为RSA预测提供了更多指标,帮助病人尽早采取保护措施。
综上所述,外周血CD4+CD25+Treg细胞和IL-10水平检测对RSA具有较高预测价值,而CD4+CD25+Treg细胞和IL-10联合检测可作为预测既往RSA孕妇再发流产的参考指标,低水平CD4+CD25+Treg细胞+IL-10的RSA孕妇流产复发的可能性更大。
| [1] |
MAHDAVIPOUR M, ZAREI S, FATEMI R, et al. Polymorphisms in the estrogen receptor beta gene and the risk of unexplained recurrent spontaneous abortion[J]. Avicenna Journal of Medical Biotechnology, 2017, 9(3): 150-154. |
| [2] |
李维宏, 牟晓玲. 黄体酮保胎治疗对不明原因复发性流产患者外周血辅助性T淋巴细胞17/调节性T淋巴细胞免疫失衡的影响研究[J]. 中国全科医学, 2015, 18(36): 4444-4449. DOI:10.3969/j.issn.1007-9572.2015.36.009 |
| [3] |
LIU Zhilan, XU Haijing, KANG Xiaomin, et al. Allogenic lymphocyte immunotherapy for unexplained recurrent spontaneous abortion: a Meta-analysis[J]. American Journal of Reproductive Immunology (New York, N.Y.:1989), 2016, 76(6): 443-453. |
| [4] |
ZONG Shanshan, LI Chunqing, LUO Chengfeng, et al. Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration[J]. Scientific Reports, 2016, 6(4): 81-85. |
| [5] |
KAUR R, GUPTA K. Endocrine dysfunction and recurrent spontaneous abortion: an overview[J]. International Journal of Applied & Basic Medical Research, 2016, 6(2): 79-83. |
| [6] |
林其德, 汪希鹏. 妇产科学新进展——原因不明复发性流产的病因病理生理机制与治疗研究进展[J]. 中国实用妇科与产科杂志, 2017, 23(1): 5-8. |
| [7] |
肖世金, 赵爱民. 复发性流产病因学研究进展[J]. 中国实用妇科与产科杂志, 2014, 30(1): 41-45. |
| [8] |
QIN W, TANG Y, TANG L, et al. Potential role of circulating microRNAs as a biomarker for unexplained recurrent spontaneous abortion[J]. American Journal of Reproductive Immunology, 2013, 70(1, SI): 22-23. |
| [9] |
ARJMAND F, GHASEMI N, MIRGHANIZADEH S A. The balance of the immune system between HLA-G and NK cells in unexplained recurrent spontaneous abortion and polymorphisms analysis[J]. Immunologic Research, 2016, 64(3): 785-790. DOI:10.1007/s12026-015-8771-9 |
| [10] |
TUNC E, TANRIVERDI N, DEMIRHAN O, et al. Chromosomal analyses of 1 510 couples who have experienced recurrent spontaneous abortions[J]. Reproductive BioMedicine Online, 2016, 32(4): 414-419. DOI:10.1016/j.rbmo.2016.01.006 |
| [11] |
PEREZA N, OSTOJIC S, KAPOVIC M, et al. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion[J]. Fertility and Sterility, 2017, 107(1): 150. DOI:10.1016/j.fertnstert.2016.10.007 |
| [12] |
HOSSEINI S, SHOKRI F, POUR S A, et al. A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion[J]. Journal of Reproductive Immunology, 2016, 116(23): 13-22. |
| [13] |
PEREZA N, OSTOJIC S, ZDRAVCEVIC M, et al. Insertion/deletion polymorphism in intron 16 of ACE gene in idiopathic recurrent spontaneous abortion: case-control study, systematic review and meta-analysis[J]. Reproductive BioMedicine Online, 2016, 32(2): 237-246. DOI:10.1016/j.rbmo.2015.11.003 |
| [14] |
DONG Xiujuan, YANG Long, WANG Hui. miR-520 promotes DNA-damage-induced trophoblast cell apoptosis by targeting PARP1 in recurrent spontaneous abortion (RSA)[J]. Gynecological Endocrinology, 2017, 33(4): 274-278. DOI:10.1080/09513590.2016.1266476 |
| [15] |
DAKHLY D M, BAYOUMI Y A, SHARKAWY M A, et al. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells[J]. International Journal of Gynecology & Obstetrics, 2016, 135(3): 324-327. |
| [16] |
FOTOOHI M, GHASEMI N, MIRGHANIZADEH S A, et al. Association between HLA-E gene polymorphism and unexplained recurrent spontaneous abortion(RSA)in Iranian women[J]. Int J Reprod Biomed (Yazd), 2016, 14(7): 477-482. DOI:10.29252/ijrm.14.7.7 |
| [17] |
SABOORI S, NOORMOHAMMADI Z, ZARE-KARIZI S. Genetic variation in vascular endothelial growth factor gene and its association with recurrent spontaneous abortion[J]. Bratislava Medical Journal-Bratislavske Lekarske Listy, 2016, 117(2): 80-86. |
| [18] |
YE L Y, GOODALL J C, ZHANG L B, et al. TCR usage, gene expression and function of two distinct FOXP3(+) Treg subsets within CD4(+)CD25(hi) T cells identified by expression of CD39 and CD45 RO[J]. Immunology and Cell Biology, 2016, 94(3): 293-305. DOI:10.1038/icb.2015.90 |
| [19] |
TALEBI A R, FESAHAT F, MANGOLI E A, et al. Relationship between sperm protamine deficiency and apoptosis in couples with unexplained repeated spontaneous abortions[J]. International Journal of Reproductive Biomedicine, 2016, 14(3): 199-204. DOI:10.29252/ijrm.14.3.199 |
| [20] |
HOMBACH A A, ABKEN H. Most do, but Some do not:CD4+CD25+T cells, but not CD4+CD25+Treg cells, are cytolytic when redirected by a chimeric antigen receptor (CAR)[J]. Cancers, 2017, 9(9): 112-115. |
| [21] |
SCHNEIDER M, MEISTER M, MULEY T. Glycodelin as diagnostic and prognostic marker and for monitoring treatment of lung diseases[J]. Pan African Medical Journal, 2016, 23(2): 13-16. |
| [22] |
GRYGOROWICZ M A, BORYCKA I S, NOWAK E A, et al. Lenalidomide potentiates CD4(+)CD25(+) Treg-related suppression of lymphoma B-cell proliferation[J]. Clinical and Experimental Medicine, 2017, 17(2): 193-207. DOI:10.1007/s10238-016-0411-8 |
| [23] |
KRONBICHLER A, BREZINA B, QUINTANA L F, et al. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: a systematic review[J]. Autoimmunity Reviews, 2016, 15(1): 38-49. DOI:10.1016/j.autrev.2015.08.010 |
| [24] |
MOTEDAYYEN H, REZAEI A, ZARNANI A. Human amniotic epithelial cells inhibit activation and pro-inflammatory cytokines production of naive CD4+T cells from women with unexplained recurrent spontaneous abortion[J]. Reproductive Biology, 2018, 18(2): 182-188. DOI:10.1016/j.repbio.2018.04.002 |
| [25] |
ARRANZ-SOLIS D, BENAVIDES J, REGIDOR-CERRILLO J, et al. Systemic and local immune responses in sheep after Neospora caninum experimental infection at early, mid and late gestation[J]. Veterinary Research, 2016, 47(1): 2-4. DOI:10.1186/s13567-015-0290-0 |
| [26] |
LOMBARDELLI L, LOGIODICE F, AGUERRE G M, et al. Interleukin-17-producing decidual CD4+T cells are not deleterious for human pregnancy when they also produce interleukin-4[J]. Clinical & Molecular Allergy, 2016, 14(1): 1-5. |
| [27] |
HOFFMAN R M, BRUMMEL S S, BRITTO P, et al. Adverse pregnancy outcomes among women who conceive on antiretroviral therapy: clinical infectious diseases an official publication of the infectious diseases[J]. Society of America, 2018, 45(2): 24-26. |
| [28] |
HADDAD L B, WALL K M, MEHTA C, et al. Trends of and factors associated with live-birth and abortion rates among HIV-positive and HIV-negative women[J]. American Journal of Obstetrics and Gynecology, 2017, 216(1): 89-92. |
| [29] |
ARMEL P, ARSÈNE H, AINA K, et al. Pregnancy rate and birth outcomes among women receiving antiretroviral therapy in Burkina Faso: a retrospective cohort study[J]. Pan African Medical Journal, 2016, 24(3): 67-71. |
| [30] |
CHRISTIANSEN O B, KOLTE A M, LARSEN E C, et al. Immunological causes of recurrent pregnancy loss[J]. Springer International Publishing, 2016, 5(2): 11-14. |
 2019, Vol. 55
2019, Vol. 55

