衰老是一个复杂的过程,随着年龄的增加,机体发生退行性改变,进而引起各种衰老相关疾病。目前,世界人口老龄化加剧,随着老龄人口的增加,衰老相关疾病逐渐增多,其中心血管疾病是导致老年人死亡的主要原因[1-2]。机体衰老后心脏会发生一系列病理性改变,如心肌纤维化、心肌肥厚、心功能不全等[3]。有研究表明,随着细胞衰老P21和P53蛋白表达增加,二者可作为衰老检测的标志性蛋白[4-6]。自噬是一种存在于细胞内的降解机制,可以清除细胞内受损的蛋白和细胞器,对细胞内稳态的调节至关重要[7-8]。衰老心肌细胞自噬调节能力降低,导致细胞内错误折叠蛋白和功能障碍的细胞器大量累积[9]。研究表明,提高心肌细胞自噬水平可以延缓心脏衰老[10]。甘露糖醛酸寡糖(M3K)是褐藻胶寡糖的一种,由来源于海洋褐藻的褐藻胶加工而成,具有抗氧化、抗炎、抗肿瘤等多种生物学活性,有重要的临床应用价值[11-13]。过氧化氢(H2O2)属于活性氧簇家族,可引起氧化应激,诱导细胞衰老,现已被广泛用于体外衰老模型的建立[14-15]。本实验采用H2O2诱导建立心肌细胞衰老模型,探讨M3K对衰老心肌细胞的保护作用及其可能机制。
1 材料和方法 1.1 实验材料M3K由中国海洋大学医药学院实验室惠赠。心肌细胞H9c2购于中国科学院上海细胞库。胎牛血清(FBS)购于美国Gibco公司,DMEM培养液购于美国Hyclone公司,P21兔单克隆抗体和P53小鼠单克隆抗体购于美国Cell Signaling Technology公司,山羊抗兔IgG二抗和山羊抗鼠IgG二抗购于武汉伊莱瑞特公司,RIPA裂解液和BCA蛋白浓度测定试剂盒购于上海碧云天公司,ECL发光液购于美国Millipore公司,逆转录试剂盒和Mix购于瑞士Roche公司。
1.2 实验方法 1.2.1 细胞培养及处理将H9c2心肌细胞接种于6孔板中,用含体积分数0.10 FBS和10 g/L青-链霉素的DMEM完全培养液,在37 ℃、含体积分数0.05 CO2的细胞培养箱中培养。实验分为5组:对照组(A组),H2O2组(B组),H2O2+M3K低剂量组(C组),H2O2+M3K中剂量组(D组),H2O2+M3K高剂量组(E组)。当细胞融合度达50%时,H2O2组、H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组细胞分别加入100 μmol/L的H2O2诱导建立衰老模型,4 h后更换新鲜DMEM完全培养液。H2O2组继续培养48 h,M3K干预各组分别加入10、50、100 mg/L的M3K培养48 h。实验过程中对照组仅进行同步换液处理。在倒置显微镜下观察,每孔随机选择3个区域进行镜下计数。收集细胞进行后续实验,所有实验均重复3次。
1.2.2 Western blot检测P21和P53蛋白表达细胞中加入RIPA裂解液提取细胞蛋白,用BCA试剂盒检测蛋白浓度。每孔加入等量的蛋白进行SDS-PAGE电泳,待溴酚蓝到达分离胶底部时转移到PVDF膜上(200 mA,90 min),用含30 g/L BSA和50 g/L脱脂奶粉的TBST溶液室温封闭2 h,加入P21和P53一抗(均1:1 000稀释),于4 ℃摇床孵育过夜;洗脱后分别加二抗(均1:5 000稀释)室温孵育2 h,用ECL发光液显影,凝胶成像系统成像后采用Quantity One软件分析条带灰度值。
1.2.3 RT-PCR检测beclin1、Atg7和LC3-Ⅱ的mRNA表达采用Trizol法提取细胞总RNA,Nanodrop测定RNA含量和浓度,应用逆转录试剂盒将mRNA逆转录为cDNA。采用RT-PCR法进行扩增,扩增条件:95 ℃、600 s;95 ℃、10 s,60 ℃、10 s,72 ℃、15 s,共40个循环;95 ℃、10 s,65 ℃、60 s,97 ℃、1 s。以β-actin作为内参,用2-ΔΔCt计算目的基因相对表达量。PCR引物序列见表 1。
| 表 1 PCR引物序列 |
|
|
应用SPSS 21.0软件进行统计学分析,计量数据以x±s表示,多组比较采用One-way ANOVA检验,组间两两比较采用LSD法。
2 结果 2.1 各组心肌细胞数量比较培养48 h后,对照组细胞数量较多,融合度达90%;H2O2组细胞数量与对照组相比明显减少;M3K干预各组细胞数量与H2O2组相比呈剂量依赖性增加,差异均有统计学意义(F=8.685,P<0.05);H2O2+M3K高剂量组细胞数量与对照组相比差异无显著性(P>0.05)。见表 2。
| 表 2 各组心肌细胞数量及P21和P53蛋白表达比较(n=3,x±s) |
|
|
与对照组相比,H2O2组心肌细胞P21和P53蛋白表达显著增加;与H2O2组相比较,M3K干预48 h后,H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组心肌细胞P21和P53蛋白表达随M3K浓度的增加而降低,差异均有显著性(F=31.507、13.111,P<0.05)。见图 1、表 2。
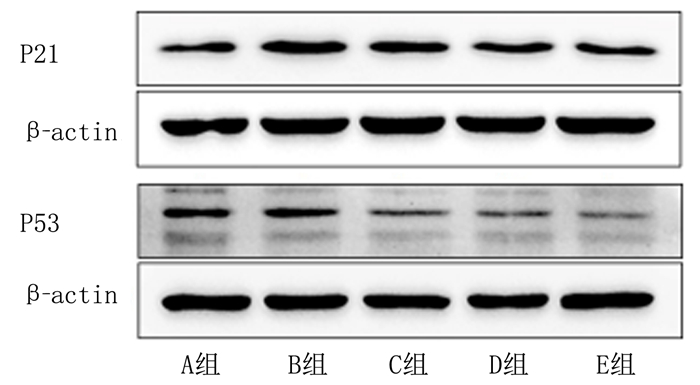 |
| 图 1 各组心肌细胞P21和P53蛋白表达Western blot检测 |
与对照组相比,H2O2组心肌细胞beclin1、Atg7和LC3-Ⅱ mRNA表达水平显著降低;与H2O2组相比,M3K干预48 h后,H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组心肌细胞beclin1、Atg7和LC3-Ⅱ mRNA表达呈浓度依赖性增加,差异均有统计学意义(F=10.134~61.041,P<0.05)。见表 3。
| 表 3 各组心肌细胞beclin1、Atg7和LC3-Ⅱ mRNA表达比较(n=3,x±s) |
|
|
衰老是导致老年人慢性疾病发生发展的重要危险因素,并且是心血管疾病发生的独立危险因素。近年来,随着老龄人口增加,衰老相关疾病给社会带来了巨大的医疗和经济负担。因此,探讨衰老相关机制、延缓衰老成为目前的研究热点[16]。H2O2诱导的氧化损伤可以较好地模拟老年人体内氧化应激,现已被广泛用于衰老模型的建立[17-18]。本实验用H2O2成功诱导了心肌细胞内氧化应激,引起细胞衰老。细胞衰老后表现为细胞周期阻滞,增殖减少。M3K属于海藻寡糖,具有多种生物学活性[11]。本研究结果显示,用M3K干预衰老心肌细胞后,细胞周期阻滞延缓,增殖加快。
P21作为细胞周期蛋白依赖性激酶抑制因子在衰老中起重要作用,它通过与细胞周期蛋白结合,导致细胞周期阻滞,阻断细胞增殖过程。细胞衰老后,P21蛋白表达增加,因此,P21可作为衰老标志物之一[19-20]。本实验采用Western blot方法检测P21蛋白的表达,观察M3K对衰老心肌细胞的影响。结果显示,H2O2诱导心肌细胞衰老后P21表达增加,而用M3K干预衰老细胞48 h可使P21蛋白表达下调,缓解衰老心肌细胞的细胞周期阻滞,进而延缓心肌细胞衰老。P53是氧化应激的关键调控分子之一[21]。P53作为“基因监护人”,可通过调节细胞周期阻滞、细胞凋亡和衰老发挥肿瘤抑制作用[22]。有研究表明,p53基因过表达小鼠可以出现早衰表型[23]。P53的表达与机体衰老呈正相关,机体衰老后,P53表达增加[24-25]。本研究结果显示,P53在H2O2诱导的衰老心肌细胞中表达增加,而M3K干预衰老细胞可使P53蛋白表达下调,表明M3K可能延缓了H2O2诱导的心肌细胞衰老。
自噬可分为巨自噬、微自噬和分子伴侣介导的自噬3种类型,其中巨自噬是最常见的一种,其自噬过程依赖于溶酶体。LC3-Ⅱ、beclin1和Atg7是自噬溶酶体形成的关键蛋白,参与溶酶体膜的形成,自噬被激活后,三者的表达增加[7, 26]。机体衰老后自噬调节能力也随之下降,细胞内受损蛋白质和细胞器大量积累,进一步加速衰老[27-28]。已有大量的研究表明,激活自噬可能延缓组织和细胞衰老[29]。为了探讨M3K延缓心肌细胞衰老是否与自噬的激活有关,本研究采用RT-PCR方法检测了自噬相关基因LC3-Ⅱ、beclin1和Atg7 mRNA的表达。结果显示,H2O2诱导的衰老细胞中LC3-Ⅱ、beclin1和Atg7 mRNA表达减少,而给予M3K处理后,细胞中LC3-Ⅱ、beclin1和Atg7 mRNA表达增加,表明M3K延缓心肌细胞衰老可能与自噬激活有关。
综上所述,M3K可以延缓H2O2诱导的H9c2心肌细胞衰老,其作用机制可能与细胞内自噬的激活有关,但其具体的分子机制还需要进一步研究。
| [1] |
SKIBSKA B, GORACA A. The protective effect of lipoic acid on selected cardiovascular diseases caused by age-related oxidative stress[J]. Oxidative Medicine and Cellular Longevity, 2015, 2015: 313021. |
| [2] |
EZZATI M, OBERMEYER Z, TZOULAKI I, et al. Contributions of risk factors and medical care to cardiovascular mortality trends[J]. Nature Reviews Cardiology, 2015, 12(9): 508-530. DOI:10.1038/nrcardio.2015.82 |
| [3] |
ALFARAS I, DI GERMANIO C, BERNIER M, et al. Pharmacological strategies to retard cardiovascular aging[J]. Circulation Research, 2016, 118(10): 1626-1642. DOI:10.1161/CIRCRESAHA.116.307475 |
| [4] |
WANG Ziling, CHEN Linbo, QIU Zhu, et al. Ginsenoside Rg1 ameliorates testicular senescence changes in Dgalinduced aging mice via antiinflammatory and antioxidative mechanisms[J]. Molecular Medicine Reports, 2018, 17(5): 6269-6276. |
| [5] |
CARRASCO G E, MORENO M, MORENO C L, et al. Increased Arf/p53 activity in stem cells, aging and cancer[J]. Aging Cell, 2017, 16(2): 219-225. DOI:10.1111/acel.12574 |
| [6] |
JIANG C, LIU G, LUCKHARDT T, et al. Serpine 1 induces alveolar type Ⅱ cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease[J]. Aging Cell, 2017, 16(5): 1114-1124. DOI:10.1111/acel.12643 |
| [7] |
LAPIERRE L R, KUMSTA C, SANDRI M, et al. Transcriptional and epigenetic regulation of autophagy in aging[J]. Autophagy, 2015, 11(6): 867-880. DOI:10.1080/15548627.2015.1034410 |
| [8] |
KIM S N, KWON H J, AKINDEHIN S, et al. Effects of epigallocatechin-3-gallate on autophagic lipolysis in adipocytes[J]. Nutrients, 2017, 9(7): 680-694. DOI:10.3390/nu9070680 |
| [9] |
SHIRAKABE A, IKEDA Y, SCIARRETTA S A, et al. Aging and autophagy in the heart[J]. Circulation Research, 2016, 118(10): 1563-1576. DOI:10.1161/CIRCRESAHA.116.307474 |
| [10] |
REN Jun, YANG Lifang, ZHU Li, et al. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling:role of Sirt1-mediated autophagy regulation[J]. Aging Cell, 2017, 16(5): 976-987. DOI:10.1111/acel.12616 |
| [11] |
ZHU Benwei, CHEN Meijuan, YIN Heng, et al. Enzymatic hydrolysis of alginate to produce oligosaccharides by a new purified endo-type alginate lyase[J]. Marine Drugs, 2016, 14(6): 108-119. DOI:10.3390/md14060108 |
| [12] |
GUO Junjie, MA Leilei, SHI Hongtao, et al. Alginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosis[J]. Marine Drugs, 2016, 14(12): 231-244. DOI:10.3390/md14120231 |
| [13] |
Guo Junjie, XU Fengqiang, LI Yonghong, et al. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis[J]. Drug Des Devel Ther, 2017, 11: 2387-2397. DOI:10.2147/DDDT.S142118 |
| [14] |
ZHU Wei, WU Yan, MENG Yifang, et al. Effect of curcumin on aging retinal pigment epithelial cells[J]. Drug Des Devel Ther, 2015, 9: 5337-5344. |
| [15] |
CHEUNG T M, GANATRA M P, FU J J, et al. The effect of stress-induced senescence on aging human cord blood-derived endothelial cells[J]. Cardiovascular Engineering and Technology, 2013, 4(2): 220-230. DOI:10.1007/s13239-013-0128-8 |
| [16] |
PERRIDON B W, LEUVENINK H G, HILLEBRANDS J L, et al. The role of hydrogen sulfide in aging and age-related pathologies[J]. Aging, 2016, 8(10): 2264-2289. DOI:10.18632/aging.101026 |
| [17] |
LI Ruilin, LU Zhaoyang, HUANG Jingjuan, et al. SRT1720, a SIRT1 specific activator, protected H2O2-induced senescent endothelium[J]. American Journal of Translational Research, 2016, 8(7): 2876-2888. |
| [18] |
SONG Zhiming, LIU Yong, HAO Baoshun, et al. Ginse-noside Rb1 prevents H2O2-induced HUVEC senescence by stimulating sirtuin-1 pathway[J]. PLoS One, 2014, 9(11): e112699. DOI:10.1371/journal.pone.0112699 |
| [19] |
HSU Y M, YIN M C. EPA or DHA enhanced oxidative stress and aging protein expression in brain of D-galactose treated mice[J]. Biomedicine (Taipei), 2016, 6(3): 23-30. |
| [20] |
JI Musi, SU Xiaohua, LIU Jizhen, et al. Comparison of naturally aging and D-galactose induced aging model in beagle dogs[J]. Experimental and Therapeutic Medicine, 2017, 14(6): 5881-5888. |
| [21] |
GAMBINO V, DE MICHELE G, VENEZIA O A, et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging[J]. Aging Cell, 2013, 12(3): 435-445. DOI:10.1111/acel.12060 |
| [22] |
QIAN Yingjuan, CHEN Xinbin. Senescence regulation by the p53 protein family[J]. Methods Mol Biol, 2013, 965: 37-61. |
| [23] |
GUEST I, ILIC Z, SCRABLE H, et al. Survival of irradiated recipient mice after transplantation of bone marrow from young, old and"early aging"mice[J]. Aging, 2015, 7(12): 1212-1222. DOI:10.18632/aging.100867 |
| [24] |
LESSEL D, WU D Y, TRUJILLO C, et al. Dysfunction of the MDM2/p53 axis is linked to premature aging[J]. Journal of Clinical Investigation, 2017, 127(10): 3598-3608. DOI:10.1172/JCI92171 |
| [25] |
BU H, WEDEL S, CAVINATO M, et al. MicroRNA regulation of oxidative stress-induced cellular senescence[J]. Oxid Med Cell Longev, 2017, 2017: 1-12. |
| [26] |
WU Yingying, CHEN Chao, YU Xiao, et al. Effects of tiaozhi granule on regulation of autophagy levels in HUVECs[J]. Evidence-Based Complementary and Alternative Medicine, 2018, 2018: 1-10. |
| [27] |
GHOSH A K, MAU T, O'BRIEN M, et al. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue[J]. Aging, 2016, 8(10): 2525-2537. DOI:10.18632/aging.101083 |
| [28] |
CARAMES B, OLMER M, KIOSSES W B, et al. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model[J]. Arthritis Rheumatol, 2015, 67(6): 1568-1576. DOI:10.1002/art.39073 |
| [29] |
PAPP D, KOVACS T, BILLES V, et al. AUTEN-67, an autophagy-enhancing drug candidate with potent antiaging and neuroprotective effects[J]. Autophagy, 2016, 12(2): 273-286. DOI:10.1080/15548627.2015.1082023 |
 2019, Vol. 55
2019, Vol. 55

