活性氧(ROS)指一组含氧基的活性化合物,包括超氧化物阴离子(O2-)、羟自由基(·OH)和过氧化氢(H2O2)等[1]。ROS能诱导心肌细胞损伤,在心肌梗死、心肌缺血再灌注损伤、心力衰竭等疾病的发生发展中发挥重要作用[2-4]。H2O2是ROS中公认的内源性和外源性诱导细胞损伤的递质,可通过脂质过氧化、DNA损伤、蛋白质结构和功能改变,最终导致细胞不可逆死亡[5],常用来诱导细胞氧化应激损伤模型[6]。7, 8-二羟基黄酮(DHF)是黄酮家族的一员,最近研究显示其是一种酪氨酸激酶受体B(TrkB)的激动剂,可通过激活下游的信号通路如蛋白激酶B(Akt)、细胞外调节蛋白激酶(Erk)等发挥神经保护及营养作用[7-9]。此外,DHF还具有抗氧化[10-12]、抗炎[13-14]、内皮细胞保护等作用[15]。DHF能通过抑制ROS产生、炎症因子释放及激活凋亡酶从而拮抗H2O2诱导的内皮细胞损伤[15],该保护作用可能与TrkB受体激活相关。但是DHF能否对H2O2诱导的心肌细胞损伤有保护作用,并无相关报道。大鼠H9c2心肌细胞来源于胚胎期心脏,被广泛用于心脏保护作用和相关信号通路的研究[16]。本研究利用H2O2诱导H9c2细胞制备氧化应激损伤模型,观察DHF对其保护作用以及PI3K/Akt信号通路是否参与该作用,为DHF应用于心血管疾病的防治提供实验依据。
1 材料和方法 1.1 试剂与仪器DHF样品购自美国TCI公司, 应用二甲基亚砜(DMSO)溶解后配制成浓度100 mmol/L储存液。体积分数0.30的H2O2购自天津市鼎盛鑫化工有限公司,四甲基偶氮唑蓝(MTT)为Solarbio(北京)公司产品,DMEM高糖培养粉为Gibco公司产品,PI3K抑制剂LY294002为APEXBIO公司产品,胎牛血清为BI公司产品,RIPA裂解液购自碧云天生物技术研究所,BCA蛋白定量检测试剂盒为Thermo公司产品,磷酸化的蛋白激酶B(p-Akt)和Akt抗体为Cell Signaling Technology公司产品,HRP-标记的二抗购自Santa Cruz公司,其他试剂均为国产分析纯。所使用仪器包括CO2培养箱、超净工作台、Olympus倒置相差显微镜、Spectra Max M5多功能酶标仪和Western显影仪等。
1.2 细胞培养将H9c2细胞置于含体积分数0.1胎牛血清、105 U/L青霉素和100 mg/L链霉素的DMEM高糖培养液中,在37 ℃、含体积分数0.05的CO2培养箱中培养。细胞达到70%~80%融合时按照1:3传代,细胞生长至对数生长期进行实验。
1.3 分组及处理方法取生长状态良好的H9c2细胞,以每孔1.5 mL接种6孔板(每孔3×105)。为观察DHF对H2O2诱导的细胞损伤的保护作用(实验1),H9c2细胞分为4组:对照组(无药物处理)、H2O2组(加入终浓度700 μmol/L H2O2孵育24 h)、DHF+H2O2组(分别加入1、5、10、20 μmol/L DHF预处理1 h,再加入700 μmol/L H2O2孵育24 h)、DHF组(加入终浓度20 μmol/L DHF孵育24 h)。为观察各组Akt蛋白水平的改变(实验2),H9c2细胞分为以下5组:对照组(无药物处理)、H2O2组(700 μmol/L H2O2孵育24 h)、DHF+H2O2组(10 μmol/L DHF预处理1 h,再加入700 μmol/L浓度的H2O2孵育24 h)、LY294002+DHF+H2O2组(首先加入10 μmol/L的LY294002孵育30 min, 其余的处理方法同DHF+H2O2组)、DHF组(加入10 μmol/L DHF孵育24 h)。为观察PI3K阻断剂LY294002对DHF保护作用的影响(实验3),H9c2细胞分5组:对照组(无药物处理)、H2O2组(应用700 μmol/L H2O2孵育24 h)、DHF+H2O2组(以10 μmol/L DHF预处理1 h后,再加入700 μmol/L H2O2孵育24 h)、LY294002+DHF+H2O2组(首先加入10 μmol/L LY294002孵育30 min, 其余处理同DHF+H2O2组)、LY294002组(加入10 μmol/L LY294002孵育24 h)。
1.4 MTT检测细胞存活率药物处理结束后,各组细胞吸出原有培养液,每孔加入5 g/L MTT 20 μL继续避光培养4 h,弃上清,加入DMSO 150 μL溶解蓝色的甲瓒颗粒,室温孵育10 min,用酶标仪测定570 nm波长处光密度(D),计算各组细胞存活率。细胞存活率(%)=(实验组D值/对照组D值) × 100%。实验重复6次,取其平均值。
1.5 Western blot检测p-Akt蛋白表达各组药物处理结束后提取蛋白,测蛋白浓度,以每孔20 μg蛋白上样,用100 g/L的SDS-PAGE凝胶电泳后转移至PDVF膜。用100 g/L BSA封闭液室温封闭1 h,再分别加入p-Akt(1:1 000)和Akt抗体(1:1 000),4 ℃孵育过夜。TBST洗膜后以HRP标记的二抗室温孵育1 h,ECL发光剂显影。用Image J软件对蛋白条带进行半定量分析,结果以p-Akt/Akt比值表示。实验重复3次。
1.6 统计学分析应用GraphPad Prism 5.0软件进行统计学处理,结果以x±s表示,多组均数比较采用单因素方差分析,两两比较采用Turkey法。P<0.05为差异有统计学意义。
2 结果 2.1 DHF对H2O2诱导H9c2心肌细胞损伤的保护作用实验1结果显示,对照组细胞存活率为(103.0±1.8)%,H2O2组细胞存活率降低至(54.1±5.8)%,提示H2O2诱导了氧化应激损伤。DHF+H2O2组分别加入1、5、10、20 μmol/L DHF预处理后,各组细胞存活率分别为(58.3±4.3)%、(71.3±2.9)%、(80.2±2.9)%和(71.5±4.4)%),其中5~20 μmol/L DHF预处理有保护作用,以10 μmol/L DHF作用最为显著(n=6, F=16.50, q=1.95~4.76, P<0.05)。结果表明,单纯应用20 μmol/L DHF处理细胞后(DHF组),细胞存活率和对照组相比无明显差异,可以排除DHF本身的毒性作用。
2.2 各组p-Akt蛋白表达比较实验2结果显示,与对照组比较,H2O2能显著减少p-Akt蛋白表达((53.7±6.2)% vs (100.0±1.7)%),而DHF预处理上调了p-Akt蛋白表达水平((94.3±2.4)% vs(53.7±6.2)%),该作用可被PI3K抑制剂LY294002所阻断((67.0±3.6)% vs(94.3±2.4)%),单独使用DHF对p-Akt蛋白表达无明显影响(n=3, F=18.67, q=6.17~10.47, P<0.01)。见图 1。
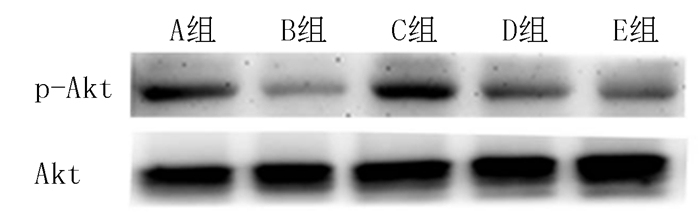 |
| A:对照组;B:H2O2组;C:DHF+H2O2组;D:LY294002+DHF+H2O2组;E:DHF组。n=3。 图 1 各组p-Akt蛋白表达Western blot检测结果 |
本文实验3结果显示,对照组、H2O2组、DHF+H2O2组、LY294002+DHF+H2O2组、LY294002组的细胞存活率分别为(99.9±2.4)%、(58.8±1.6)%、(84.5±3.7)%、(64.1±1.9)%、(89.8±1.2)%。与对照组比较,H2O2组细胞存活率明显降低,该作用可被10 μmol/L DHF所拮抗,而DHF的保护作用可部分被LY294002所阻断(n=6, F=44.75, q=8.52~17.16, P<0.01)。而单独加入LY294002后,细胞存活率和对照组比较差异无显著性(P>0.05),可排除LY294002的毒性作用。
3 讨论氧化应激损伤造成的细胞凋亡在缺血性心脏病的病程进展中发挥重要作用。DHF是一种天然存在的黄酮类化合物,除了自由基清除作用外,也能与神经细胞膜上的TrkB受体结合,发挥神经保护和营养作用。本实验目的是探讨DHF对心肌细胞氧化应激损伤是否具有保护作用,以及可能参与的信号通路。
本文研究结果表明,DHF能明显抑制H2O2诱导的H9c2心肌细胞损伤,DHF的保护作用可能和其激活PI3K/Akt信号通路密切相关。H2O2诱导的H9c2细胞损伤机制复杂,H2O2可能通过诱导脂质过氧化、DNA损伤和蛋白质结构和功能异常,导致氧化应激损伤[5]。此外,H2O2还可通过诱导线粒体途径的凋亡以及下调Akt信号蛋白表达等[17],导致细胞不可逆的死亡。
DHF对H9c2细胞产生保护作用的可能机制是:H9c2细胞膜存在TrkB受体[18],DHF结合该受体后可能激活相关信号通路如Akt等[19]。另外,由于DHF分子结构中的两个羟基能够直接清除自由基,DHF也能提高细胞内抗氧化酶(如SOD)的活性[20],因此,DHF对H9c2细胞的保护作用可能与其抗氧化特性密切相关。
本研究观察了PI3K/Akt通路是否参与DHF的保护作用。PI3K/Akt是细胞内一条重要的信号通路,可参与细胞的生长、增殖、分化以及凋亡等活动[21-22]。PI3K可导致下游Akt磷酸化,后者通过调控下游的相关蛋白如血红素加氧酶1和Bcl-2/Bax等的表达,发挥抗氧化与抗凋亡作用[23-24]。有研究结果显示,某些抗氧化剂(如石斛兰和牛奶树碱)在H9c2细胞氧化损伤模型中,可通过激活Akt信号通路发挥重要的保护作用[25-28]。我们的前期研究也发现,DHF能够通过上调p-Akt蛋白的表达对抗6-OHDA诱导的PC12细胞损伤[29]。本研究结果显示,DHF可对抗H2O2诱导的Akt失活,并且LY294002预处理也部分拮抗DHF的保护作用, 提示DHF的保护作用部分与激活PI3K/Akt信号通路有关。我们后续实验将进一步探讨DHF的保护作用机制。
| [1] |
SONG Yi, NI Yuanying, HU Xiaosong, et al. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide[J]. International Journal of Biological Macromolecules, 2015, 81(81): 41-48. |
| [2] |
REN Lin, WANG Qian, CHEN Yu, et al. Involvement of microRNA-133a in the protective effect of Hydrogen sulfide against ischemia/reperfusion-induced endoplasmic reticulum stress and cardiomyocyte apoptosis[J]. Pharmacology, 2018, 103(1/2): 1-9. |
| [3] |
HE Weilai, CHE Hong, JIN Chaolong, et al. Effects of miR-23b on hypoxia-induced cardiomyocytes apoptosis[J]. Biomedicine & Pharmacotherapy, 2017, 96(96): 812-817. |
| [4] |
YAO T, FUJIMURA T, MURAYAMA K, et al. Oxidative stress-responsive apoptosis inducing protein(ORAIP)plays a critical role in high glucose-induced apoptosis in rat cardiac myocytes and murine pancreatic β-cells[J]. Cells (Basel, Switzerland), 2017, 6(4): 35-43. |
| [5] |
DRÖGE W. Free radicals in the physiological control of cell function[J]. Physiological Reviews, 2002, 82(1): 47-95. DOI:10.1152/physrev.00018.2001 |
| [6] |
NIU Tong, JIN Liuzhong, NIU Shizhen, et al. Lycium barbarum polysaccharides alleviates oxidative damage induced by H2O2 through down-regulating microRNA-194 in PC12 and SH-SY5Y cells[J]. Cellular Physiology and Biochemistry, 2018, 50(2): 460-472. DOI:10.1159/000494159 |
| [7] |
JANG S W, LIU X, YEPES M, et al. A selective TrkB agonist with potent neurotrophic activities by 7, 8-dihydroxyflavone[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(6): 2687-2692. DOI:10.1073/pnas.0913572107 |
| [8] |
AYTAN N, CHOI J K, CARRERAS I, et al. Protective effects of 7, 8-dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of Alzheimer's disease[J]. European Journal of Pharmacology, 2018, 828(828): 9-17. |
| [9] |
HUANG H M, HUANG C C, TSAI M H, et al. Systemic 7, 8-Dihydroxyflavone treatment protects immature retinas against hypoxic-ischemic injury via muller Glia regeneration and MAPK/ERK activation[J]. Investigative Ophthalmology & Visual Science, 2018, 59(7): 3124-3135. |
| [10] |
HAN Xiaohua, ZHU Shaolei, WANG Bingxiang, et al. Antioxidant action of 7, 8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cytotoxicity[J]. Neurochemistry International, 2014, 64(64): 18-23. |
| [11] |
MA Rui, ZHANG Jisheng, LIU Xiaoyu, et al. 7, 8-DHF treatment induces Cyr61 expression to suppress hypoxia induced ER stress in HK-2 cells[J]. BioMed Research International, 2016, 2016: 5029797. DOI:10.1155/2016/5029797 |
| [12] |
KANG J S, CHOI I W, HAN M H, et al. The cytoprotective effects of 7, 8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts[J]. International Journal of Molecular Medicine, 2015, 36(2): 501-510. DOI:10.3892/ijmm.2015.2256 |
| [13] |
JIN Zhen, YANG Yaozhi, CHEN Jianxin, et al. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7, 8-dihydroxyflavone[J]. Journal of Pharmacy and Pharmacology, 2017, 69(7): 865-874. DOI:10.1111/jphp.12714 |
| [14] |
PARK H Y, PARK C, HWANG H J, et al. 7, 8-Dihydroxyflavone attenuates the release of pro-inflammatory mediators and cytokines in lipopolysaccharide-stimulated BV2 microglial cells through the suppression of the NF-kappa B and MAPK signaling pathways[J]. International Journal of Molecular Medicine, 2014, 33(4): 1027-1034. DOI:10.3892/ijmm.2014.1652 |
| [15] |
WANG Bingxiang, ZHANG Qian, YAO Ruyong, et al. 7, 8-Dihydroxyflavone protects an endothelial cell line from H2O2 damage[J]. PLoS One, 2015, 10(8): e0135345. DOI:10.1371/journal.pone.0135345 |
| [16] |
KUZNETSOV A V, JAVADOV S, SICKINGER S A, et al. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation[J]. Biochimica et Biophysica Acta-Molecular Cell Research, 2015, 1853(2): 276-284. DOI:10.1016/j.bbamcr.2014.11.015 |
| [17] |
JUN H O, KIM D H, LEE S W, et al. Clusterin protects H9c2 cardiomyocytes from oxidative stress-induced apoptosis via Akt/GSK-3β signaling pathway[J]. Experimental & Molecular Medicine, 2011, 43(1): 53-61. |
| [18] |
HANG Pengzhou, ZHAO Jing, SUN Li, et al. Brain-derived neurotrophic factor attenuates doxorubicin-induced cardiac dysfunction through activating Akt signalling in rats[J]. Journal of Cellular and Molecular Medicine, 2017, 21(4): 685-696. DOI:10.1111/jcmm.13012 |
| [19] |
TECUATL C, HERRRERA-LOPEZ G, MARTIN-AVILA A, et al. TrkB-mediated activation of the phosphatidylinositol-3-kinase/Akt cascade reduces the damage inflicted by oxygen-glucose deprivation in area CA3 of the rat hippocampus[J]. European Journal of Neuroscience, 2018, 47(9): 1096-1109. DOI:10.1111/ejn.13880 |
| [20] |
CHEN J, CHUA K W, CHUA C C, et al. Antioxidant activity of 7, 8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity[J]. Neuroscience Letters, 2011, 499(3): 181-185. DOI:10.1016/j.neulet.2011.05.054 |
| [21] |
HSU H S, LIU C C, LIN J H, et al. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis[J]. Scientific Reports, 2017, 7(1): 14272. DOI:10.1038/s41598-017-14612-5 |
| [22] |
LV Danni, BAI Zhixia, YANG Libin, et al. Lipid emulsion reverses bupivacaine-induced apoptosis of h9c2 cardiomyocytes: PI3K/Akt/GSK-3 beta signaling pathway[J]. Environmental Toxicology and Pharmacology, 2016, 42(42): 85-91. |
| [23] |
JIANG Youqin, CHANG Guanglei, WANG Ying, et al. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway[J]. Cellular Physiology and Biochemistry, 2016, 39(1): 407-421. DOI:10.1159/000445634 |
| [24] |
WANG Z, SU G, ZHANG Z, et al. 2.5-Hydroxyl-protopanaxatriol protects against H2O2-induced H9c2 cardiomyocytes injury via PI3K/Akt pathway and apoptotic protein down-regulation[J]. Biomedicine Pharmaco Therapy, 2018(99): 33-42. |
| [25] |
KIM D E, KIM B, SHIN H S, et al. The protective effect of hispidin against Hydrogen peroxide-induced apoptosisin H9c2 cardioyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway[J]. Experimental Cell Research, 2014, 327(2): 264-275. |
| [26] |
ZHANG Jingyi, GUO Ying, SI Jinping, et al. A polysaccharide of Dendrobium officinale ameliorates H2O2-induced apoptosis in H9c2 cardiomyocytes via PI3K/AKT and MAPK pathways[J]. International Journal of Biological Macromolecules, 2017, 104(A): 1-10. |
| [27] |
CHU Donghai, ZHANG Zhenqiu. Trichosanthis pericarpium aqueous extract protects H9c2 cardiomyocytes from hypoxia/reoxygenation injury by regulating PI3K/Akt/NO pathway[J]. Molecules, 2018, 23(10). DOI:10.3390/molecules23102409 |
| [28] |
WANG Yi, CHE Jianbo, ZHAO Hui, et al. Platycodin D inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury[J]. Biochemical and Biophysical Research Communications, 2018, 503(4): 3219-3224. DOI:10.1016/j.bbrc.2018.08.129 |
| [29] |
HAN Xiaohua, CHENG Mengnan, CHEN Lei, et al. 7, 8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cell death through modulating PI3K/Akt and JNK pathways[J]. Neuroscience Letters, 2014, 581(13/CX/3): 85-88. |
 2019, Vol. 55
2019, Vol. 55

